Long-term follow-up in common variable immunodeficiency: the pediatric-onset and adult-onset landscape
- PMID: 37435172
- PMCID: PMC10332319
- DOI: 10.3389/fped.2023.1125994
Long-term follow-up in common variable immunodeficiency: the pediatric-onset and adult-onset landscape
Abstract
Introduction: The primary aim of this study is to investigate the evolution of the clinical and laboratory characteristics during the time in a longitudinal cohort of pediatric-onset and adult-onset Common Variable Immunodeficiency (CVID) patients in order to identify early predictive features of the disease and immune dysregulation complications.
Methods: This is a retrospective-prospective monocentric longitudinal study spanning from 1984 to the end of 2021. The data of pediatric-onset vs. adult-onset patients have been compared for immunological features and for infectious and non-infectious complications assessed at diagnosis and follow-up.
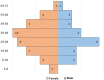
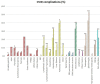
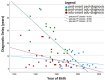
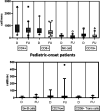
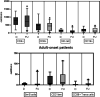
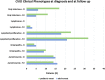
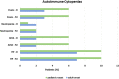
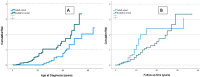
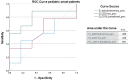
Results: Seventy-three CVID patients have been enrolled, with a mean of 10.0 years (SD ± 8.17) of prospective follow-up. At diagnosis, infections were observed in 89.0% of patients and immune dysregulation in 42.5% of patients. At diagnosis, 38.6% of pediatric-onset and 20.7% of adult-onset patients presented with only infections. Polyclonal lymphoid proliferation (62.1%) and autoimmunity (51.7%) were more prevalent in the adult-onset than in the pediatric-onset group (polyclonal lymphoid proliferation 52.3% and autoimmunity 31.8%, respectively). Enteropathy was present in 9.1% of pediatric-onset and 17.2% of adult-onset patients. The prevalence of polyclonal lymphoid proliferation increased during follow-up more in pediatric-onset patients (diagnosis 52.3%-follow-up 72.7%) than in adult-onset patients (diagnosis 62.1%-follow-up 72.7%). The cumulative risk to develop immune dysregulation increases according to the time of disease and the time of diagnostic delay. At the same age, pediatric-onset patients have roughly double the risk of having a complication due to immune dysregulation than adult-onset patients, and it increases with diagnostic delay. The analysis of lymphocyte subsets in the pediatric-onset group showed that CD21 low B cells at diagnosis may be a reliable prognostic marker for the development of immune dysregulation during follow-up, as the ROC curve analysis showed (AUC = 0.796). In the adult-onset group, the percentage of transitional B cells measured at diagnosis showed a significant accuracy (ROC AUC = 0.625) in identifying patients at risk of developing immune dysregulation.
Discussion: The longitudinal evaluation of lymphocyte subsets combined with clinical phenotype can improve the prediction of lymphoid proliferation and allow experts to achieve early detection and better management of such complex disorder.
Keywords: CD21low B cells; autoimmunity; common variable immunodeficiency (CVID); cytopenia; immune dysregulation; inborn errors of immunity; lymphoid proliferation.
© 2023 Carrabba, Salvi, Baselli, Serafino, Zarantonello, Trombetta, Pietrogrande, Fabio and Dellepiane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Two Sides of the Same Coin: Pediatric-Onset and Adult-Onset Common Variable Immune Deficiency.J Clin Immunol. 2017 Aug;37(6):592-602. doi: 10.1007/s10875-017-0415-5. Epub 2017 Jul 28. J Clin Immunol. 2017. PMID: 28755066
-
The Immune Dysregulation of Common Variable Immunodeficiency Disorders.Immunol Lett. 2021 Feb;230:21-26. doi: 10.1016/j.imlet.2020.12.002. Epub 2020 Dec 14. Immunol Lett. 2021. PMID: 33333111 Review.
-
Common Variable Immunodeficiency and Autoimmune Diseases: A Retrospective Study of 95 Adult Patients in a Single Tertiary Care Center.Front Immunol. 2021 Jul 5;12:652487. doi: 10.3389/fimmu.2021.652487. eCollection 2021. Front Immunol. 2021. PMID: 34290696 Free PMC article.
-
Lymphocyte Subset Abnormalities in Pediatric-Onset Common Variable Immunodeficiency.Int Arch Allergy Immunol. 2020;181(3):228-237. doi: 10.1159/000504598. Epub 2020 Jan 3. Int Arch Allergy Immunol. 2020. PMID: 31901904
-
The pediatric common variable immunodeficiency - from genetics to therapy: a review.Eur J Pediatr. 2022 Apr;181(4):1371-1383. doi: 10.1007/s00431-021-04287-6. Epub 2021 Dec 23. Eur J Pediatr. 2022. PMID: 34939152 Free PMC article. Review.
Cited by
-
The Burden of Non-Infectious Organ-Specific Immunopathology in Pediatric Common Variable Immunodeficiency.Int J Mol Sci. 2025 Mar 15;26(6):2653. doi: 10.3390/ijms26062653. Int J Mol Sci. 2025. PMID: 40141295 Free PMC article. Review.
-
Immunogenetic Landscape in Pediatric Common Variable Immunodeficiency.Int J Mol Sci. 2024 Sep 17;25(18):9999. doi: 10.3390/ijms25189999. Int J Mol Sci. 2024. PMID: 39337487 Free PMC article. Review.
-
Fecal microbiota transplantation in a patient with chronic diarrhea and primary and secondary immunodeficiency (common variable immunodeficiency and splenectomy).Front Cell Infect Microbiol. 2024 Sep 30;14:1456672. doi: 10.3389/fcimb.2024.1456672. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39403201 Free PMC article.
-
Monogenic Common Variable Immunodeficiency (Mo-CVID) Score for Optimizing the Genetic Diagnosis in Pediatric CVID Cohort.Eur J Immunol. 2025 Mar;55(3):e202451433. doi: 10.1002/eji.202451433. Eur J Immunol. 2025. PMID: 40079712 Free PMC article.
-
Chronic Kidney Disease in Common Variable Immunodeficiency: a Multicenter Study.J Clin Immunol. 2025 May 23;45(1):97. doi: 10.1007/s10875-025-01890-2. J Clin Immunol. 2025. PMID: 40407942 Free PMC article.
References
-
- European Society for Immunodeficiencies (ESID). Registry Working Party Diagnosis Criteria (2019). https://esid.org/Working-Parties/Registry-Working-Party/Diagnosis-criteria (Accessed May 28, 2022).

