Serum osteopontin can improve papillary thyroid cancer risk assessment of Bethesda III thyroid nodules: a preliminary study
- PMID: 37435186
- PMCID: PMC10265541
- DOI: 10.1530/EO-21-0005
Serum osteopontin can improve papillary thyroid cancer risk assessment of Bethesda III thyroid nodules: a preliminary study
Abstract
Objective: Thyroid cancer can be detected in 5-10% of patients with thyroid nodules. Management may be a challenge if fine-needle aspiration biopsy yields Bethesda III findings. Most of these cases undergo surgery and are ultimately found benign. Our aim was to evaluate whether serum osteopontin can accurately estimate thyroid cancer risk in cases with cytologically Bethesda III thyroid nodules and, thereby, decrease the number of unnecessary surgical interventions.
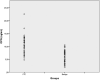
Design and methods: We obtained blood samples of cases with repeated cytologically Bethesda III thyroid nodules before surgery, and followed up the pathology results after thyroidectomy. We evaluated serum osteopontin from 36 patients with papillary thyroid cancer and compared them with 40 benign cases.
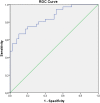
Results: Serum osteopontin levels in patients with papillary thyroid cancer are significantly higher than in benign cases (mean serum osteopontin: 10.48 ± 3.51 ng/mL vs6.14 ± 2.29 ng/mL, P < 0.001). The area under the receiver operating characteristics curve was 0.851, suggesting that serum osteopontin could have considerable discriminative performance.
Conclusions: In our preliminary study, high serum osteopontin levels can predict the risk of papillary thyroid cancer in thyroid nodules with Bethesda III cytology. Further studies are necessary to confirm these findings.
Keywords: osteopontin; thyroid cancer; thyroid nodule.
© The author.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
Similar articles
-
Repeat Fine Needle Aspiration Cytology Refines the Selection of Thyroid Nodules for Afirma Gene Expression Classifier Testing.Thyroid. 2021 Aug;31(8):1253-1263. doi: 10.1089/thy.2020.0969. Epub 2021 Jun 22. Thyroid. 2021. PMID: 33813868 Free PMC article.
-
Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study.JAMA Oncol. 2019 Feb 1;5(2):204-212. doi: 10.1001/jamaoncol.2018.4616. JAMA Oncol. 2019. PMID: 30419129 Free PMC article.
-
Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology.BMC Endocr Disord. 2020 Apr 15;20(1):48. doi: 10.1186/s12902-020-0530-9. BMC Endocr Disord. 2020. PMID: 32293401 Free PMC article.
-
Ultrasound characteristics of thyroid nodules facilitate interpretation of the malignant risk of Bethesda system III/IV thyroid nodules and inform therapeutic schedule.Diagn Cytopathol. 2019 Sep;47(9):881-889. doi: 10.1002/dc.24248. Epub 2019 Jun 18. Diagn Cytopathol. 2019. PMID: 31211509 Free PMC article.
-
Repeat Fine-Needle Aspiration With Molecular Analysis in Management of Indeterminate Thyroid Nodules.Otolaryngol Head Neck Surg. 2023 Apr;168(4):738-744. doi: 10.1177/01945998221093527. Epub 2023 Feb 5. Otolaryngol Head Neck Surg. 2023. PMID: 35412868 Review.
References
-
- Chernaya G, Mikhno N, Khabalova T, Svyatchenko S, Mostovich L, Shevchenko S, Gulyaeva L. 2018. The expression profile of integrin receptors and osteopontin in thyroid malignancies varies depending on the tumor progression rate and presence of BRAF V600E mutation. Surgical Oncology 27 702–708. (10.1016/j.suronc.2018.09.007) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials