Bioequivalence Studies of Sildenafil Citrate Orodispersible Film Administered with and without Water vs ViagraⓇ Film-Coated Tablets in Healthy Male Volunteers
- PMID: 37435189
- PMCID: PMC10331808
- DOI: 10.1016/j.curtheres.2023.100708
Bioequivalence Studies of Sildenafil Citrate Orodispersible Film Administered with and without Water vs ViagraⓇ Film-Coated Tablets in Healthy Male Volunteers
Abstract
Background: Orodispersible film (ODF) formulation offers ease of use, convenience of administration, and other advantages, especially for patients who have difficulty in swallowing or are on liquid restriction compared with conventional oral formulations for the treatment of erectile dysfunction.
Objectives: These studies compared the bioequivalence of 50 mg sildenafil citrate ODF formulation (test drug) with the marketed 50 mg sildenafil citrate film-coated tablet (FCT) (ViagraⓇ; Pfizer, New York, NY) (reference drug), with and without water in 2 randomized cross-over studies.
Methods: Two randomized cross-over studies were conducted. The first study explored the bioequivalence of test drug administered with and without water compared with the reference drug with water. The second study investigated the bioequivalence of test drug, without water, compared with the reference drug with water. Forty-two and 80 healthy male volunteers were recruited in the first and second study, respectively. All volunteers fasted for 10 hours pre-dose. A 1-day washout period between doses was observed. Blood samples were collected at both before (up to 120 minutes before dosing) and after dosing (at different intervals up to 14 hours) stages. Statistical analyses on pharmacokinetic parameters were performed. Safety and tolerability for both the formulations were evaluated.
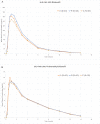
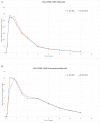
Results: In the first study, bioequivalence was demonstrated for sildenafil citrate ODF administered with water when compared with the ViagraⓇ FCT. The ratios of adjusted geometric means (90% confidence interval (CI)) were maximum plasma concentration: 1.02 (94.91-108.78) and area under the plasma concentration-time curve: 1.09 (104.49-113.21) for sildenafil citrate ODF administered with water vs ViagraⓇ FCT. These ratios were within the bioequivalence acceptance range of 80% to 125%, indicating that the bioequivalence criteria were met. The pharmacokinetic parameters for the second study also showed bioequivalence for sildenafil citrate ODF (without water) compared with ViagraⓇ FCT. The ratios of adjusted geometric means (90% CI) were maximum plasma concentration: 1.02 (95.47-109.36) and area under the plasma concentration-time curve: 1.06 (103.42-108.40) for sildenafil citrate ODF administered without water vs ViagraⓇ FCT. Adverse events in both the studies occurred at similar rates for the 2 formulations and were mild in intensity.
Conclusions: These results suggest that the new ODF formulation can be used interchangeably with the marketed FCT formulation. Sildenafil citrate ODF administered with and without water met bioequivalence criteria compared with ViagraⓇ FCT administered with water under fasted conditions in healthy adult male volunteers. The new ODF formulation can be used as a suitable alternative to the conventional oral solid dosage form.
Keywords: Bioequivalence; Erectile dysfunction; Film-coated tablets; Orodispersible film; Pharmacokinetic parameters; Sildenafil.
© 2023 Viatris.
Conflict of interest statement
A. Shaw, N. Summers, T. Hassan, T. Lawrence, S. Tarr, M. Liu, and S. Hackley are employees of Viatris Inc and hold stocks. S. Vignesh, T. Yan, and V. Daggumati are employees of Viatris Inc. J. Rondon is an employee of CPMI. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Figures
Similar articles
-
Next-generation pharmaceuticals: the rise of sildenafil citrate ODF for the treatment of men with erectile dysfunction.Ther Deliv. 2025 Apr;16(4):365-378. doi: 10.1080/20415990.2024.2445501. Epub 2025 Jan 12. Ther Deliv. 2025. PMID: 39801170 Free PMC article. Review.
-
Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers.Drug Des Devel Ther. 2017 Apr 11;11:1183-1192. doi: 10.2147/DDDT.S124034. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28442892 Free PMC article. Clinical Trial.
-
Pharmacokinetic comparison of an orally disintegrating film formulation with a film-coated tablet formulation of sildenafil in healthy Korean subjects: a randomized, open-label, single-dose, 2-period crossover study.Clin Ther. 2013 Mar;35(3):205-14. doi: 10.1016/j.clinthera.2013.02.006. Clin Ther. 2013. PMID: 23497759 Clinical Trial.
-
Comparison of tadalafil pharmacokinetics after administration of a new orodispersible film versus a film-coated tablet.Drug Des Devel Ther. 2018 Apr 20;12:935-942. doi: 10.2147/DDDT.S155040. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29719379 Free PMC article. Clinical Trial.
-
Phosphodiesterase Type 5 Inhibitors for the Treatment of Erectile Dysfunction: Pharmacology and Clinical Impact of the Sildenafil Citrate Orodispersible Tablet Formulation.Clin Ther. 2017 Feb;39(2):370-377. doi: 10.1016/j.clinthera.2017.01.001. Epub 2017 Jan 28. Clin Ther. 2017. PMID: 28139291 Review.
Cited by
-
Next-generation pharmaceuticals: the rise of sildenafil citrate ODF for the treatment of men with erectile dysfunction.Ther Deliv. 2025 Apr;16(4):365-378. doi: 10.1080/20415990.2024.2445501. Epub 2025 Jan 12. Ther Deliv. 2025. PMID: 39801170 Free PMC article. Review.
-
Effects of Postprandial Factors and Second Meal Intake Time on Bioequivalence Investigation of Tadalafil-Loaded Orodispersible Films in Human Volunteers.Pharmaceutics. 2024 Jul 9;16(7):915. doi: 10.3390/pharmaceutics16070915. Pharmaceutics. 2024. PMID: 39065611 Free PMC article.
References
-
- Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63(4):811–859. - PubMed
-
- Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7(1 Pt 2):445–475. - PubMed
-
- Artom N, et al. Prevalence of Erectile Dysfunction in a Cohort of Italian Hypertensive Subjects. Clinical and Experimental Hypertension. 2016;38(2):143. - PubMed
-
- European Association of Urology. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Prema-ture Ejaculation. http://uroweb.org/wp-content/uploads/14-Male-Sexual-Dysfunction_LR1.pdf. Accessed August 1, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

