SAKK 21/12: a phase II trial of transdermal CR1447 in breast cancer patients
- PMID: 37435469
- PMCID: PMC10259314
- DOI: 10.1530/EO-21-0009
SAKK 21/12: a phase II trial of transdermal CR1447 in breast cancer patients
Abstract
Objective: CR1447, a novel transdermal formulation of 4-hydroxytestosterone, has aromatase-inhibiting and androgen receptor (AR)-modulating properties (IC504.4 nM) with antitumor effects against AR-positive tumor cells in vitro. This trial investigated the efficacy and safety of CR1447 for patients with metastatic estrogen receptor-positive (A) and AR-positive triple-negative breast cancers (B).
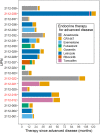
Design and methods: (A) included patients with at most one prior endocrine therapy line without progression ≥6 months, whereas (B) included patients with ≤2 prior chemotherapy lines, all displaying advanced signs of disease. The primary endpoint was disease control at week 24 (DC24). The null hypothesis was DC24 ≤30% (A) and ≤15% (B). Thirty-seven patients were recruited (29 in (A) and 8 in (B)); accrual was stopped following an interim analysis demonstrating futility in (A) and slow accrual in (B).
Results: DC24 was attained in 5/21 (95% CI: 8.2-47.2) patients in (A) and none in (B). The median progression-free survival was 5.1 months (95% CI: 2.5-5.6) in (A) and 2.5 months (95% CI: 0.7-2.6) in (B). The median overall survival was 24.6 months (95% CI: 22.9-not applicable) in (A) and 10.8 months (95% CI: 3.3-10.9) in (B). CR1447 had a favorable safety profile without treatment-related grade 3-5 toxicities in (A). Especially no side effects linked to androgenic effects were observed.
Conclusions: Despite this trial being negative, the 24% DC24 rate in a second-line setting, and the prolonged partial response experienced by a patient, indicate activity. Further evaluation of CR1447 in endocrine-sensitive patients or combination trials appears warranted.
Keywords: CR1447; androgen receptor; endocrine therapy; estrogen receptor; metastatic breast cancer.
© The authors.
Conflict of interest statement
W S is a scientific advisor for CURADIS. The other authors have nothing to disclose.
Figures

References
-
- Bernhard J, Castiglione-Gertsch M, Schmitz S-F, Thürlimann B, Cavalli F, Morant R, Fey MF, Bonnefoi H, Goldhirsch A, Hürny C.1999Quality of life in postmenopausal patients with breast cancer after failure of tamoxifen: formestane versus megestrol acetate as second-line hormonal treatment. European Journal of Cancer 35913–920. (10.1016/S0959-8049(9900028-3) - DOI - PubMed
-
- Boni C, Pagano M, Panebianco M, Bologna A, Sierra NMA, Gnoni R, Formisano D, Bisagni G.2014Therapeutic activity of testosterone in metastatic breast cancer. Anticancer Research 341287–1290. (available at: http://www.ncbi.nlm.nih.gov/pubmed/24596374) - PubMed
-
- Buzdar AU, Jones SE, Vogel CL, Wolter J, Plourde P, Webster A.1997A phase III trial comparing anastrozole (1 and 10 milligrams), a potent and selective aromatase inhibitor, with megestrol acetate in postmenopausal women with advanced breast carcinoma. Arimidex Study Group. Cancer 79730–739. (10.1002/(SICI)1097-0142(19970215)79:4<730::AID-CNCR10>3.0.CO;2-0) - DOI - PubMed
-
- Byrne MJ, Gebski V, Forbes J, Tattersall MH, Simes RJ, Coates AS, Dewar J, Lunn M, Flower C, Gill PGet al.1997Medroxyprogesterone acetate addition or substitution for tamoxifen in advanced tamoxifen-resistant breast cancer: a phase III randomized trial. Australian-New Zealand Breast Cancer Trials Group. Journal of Clinical Oncology 153141–3148. (10.1200/JCO.1997.15.9.3141) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
