A Combination of Ex Vivo and In Vivo Strategies for Evaluating How Much New Oral Anticoagulants Exacerbate Experimental Intracerebral Bleeding
- PMID: 37435564
- PMCID: PMC10332909
- DOI: 10.1055/s-0043-1770782
A Combination of Ex Vivo and In Vivo Strategies for Evaluating How Much New Oral Anticoagulants Exacerbate Experimental Intracerebral Bleeding
Abstract
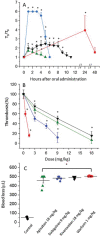
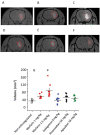
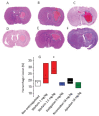
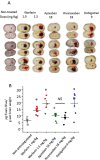
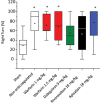
Background Intracerebral hemorrhage is the most serious complication of anticoagulant therapy but the effects of different types of oral anticoagulants on the expansion of these hemorrhages are still unclear. Clinical studies have revealed controversial results; more robust and long-term clinical evaluations are necessary to define their outcomes. An alternative is to test the effect of these drugs in experimental models of intracerebral bleeding induced in animals. Aims To test new oral anticoagulants (dabigatran etexilate, rivaroxaban, and apixaban) in an experimental model of intracerebral hemorrhage induced by collagenase injection into the brain striatum of rats. Warfarin was used for comparison. Methods Ex vivo anticoagulant assays and an experimental model of venous thrombosis were employed to determine the doses and periods of time required for the anticoagulants to achieve their maximum effects. Subsequently, volumes of brain hematoma were evaluated after administration of the anticoagulants, using these same parameters. Volumes of brain hematoma were evaluated by magnetic resonance imaging, H&E (hematoxylin and eosin) staining, and Evans blue extravasation. Neuromotor function was assessed by the elevated body swing test. Results and Conclusions The new oral anticoagulants did not increase intracranial bleeding compared with control animals, while warfarin markedly favored expansion of the hematomas, as revealed by magnetic resonance imaging and H&E staining. Dabigatran etexilate caused a modest but statistically significant increase in Evans blue extravasation. We did not observe significant differences in elevated body swing tests among the experimental groups. The new oral anticoagulants may provide a better control over a brain hemorrhage than warfarin.
Keywords: apixaban; dabigatran; intracerebral hemorrhage; rivaroxaban; warfarin.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. ( https://creativecommons.org/licenses/by/4.0/ ).
Conflict of interest statement
Conflicts of Interest None declared.
Figures
Similar articles
-
In Models of Intracerebral Hemorrhage, Rivaroxaban is Superior to Warfarin to Limit Blood Brain Barrier Disruption and Hematoma Expansion.Curr Neurovasc Res. 2017;14(2):96-103. doi: 10.2174/1567202613666161216150835. Curr Neurovasc Res. 2017. PMID: 27993122
-
Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation.J Am Coll Cardiol. 2012 Aug 21;60(8):738-46. doi: 10.1016/j.jacc.2012.03.019. Epub 2012 May 9. J Am Coll Cardiol. 2012. PMID: 22575324
-
Volume and Characteristics of Intracerebral Hemorrhage with Direct Oral Anticoagulants in Comparison with Warfarin .Cerebrovasc Dis Extra. 2017;7(1):62-71. doi: 10.1159/000462985. Epub 2017 Apr 3. Cerebrovasc Dis Extra. 2017. PMID: 28376486 Free PMC article.
-
Bleeding with dabigatran, rivaroxaban, apixaban. No antidote, and little clinical experience.Prescrire Int. 2013 Jun;22(139):155-9. Prescrire Int. 2013. PMID: 23866358 Review.
-
Direct Oral Anticoagulants Versus Vitamin K Antagonists in Real-life Patients With Atrial Fibrillation. A Systematic Review and Meta-analysis.Rev Esp Cardiol (Engl Ed). 2019 Apr;72(4):305-316. doi: 10.1016/j.rec.2018.03.009. Epub 2018 Mar 30. Rev Esp Cardiol (Engl Ed). 2019. PMID: 29606361 English, Spanish.
References
-
- Kearon C, Kahn S R, Agnelli G, Goldhaber S, Raskob G E, Comerota A J. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(06):454S–545S. - PubMed
-
- Steffel J, Braunwald E.Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism Eur Heart J 201132161968–1976., 1976a - PubMed
-
- Rincon F, Mayer S A. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19(01):95–102. - PubMed
-
- Canadian PCC Registry (CanPro) Investigators . Dowlatshahi D, Butcher K S, Asdaghi N. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke. 2012;43(07):1812–1817. - PubMed
-
- CHANT Investigators . Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P. Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. 2008;39(11):2993–2996. - PubMed
LinkOut - more resources
Full Text Sources

