Warfarin pharmacogenetics in a black Zimbabwean cohort: an observational prospective study
- PMID: 37435666
- PMCID: PMC10621760
- DOI: 10.2217/pgs-2023-0089
Warfarin pharmacogenetics in a black Zimbabwean cohort: an observational prospective study
Abstract
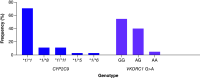
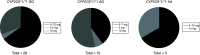
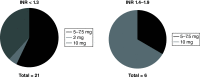
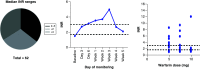
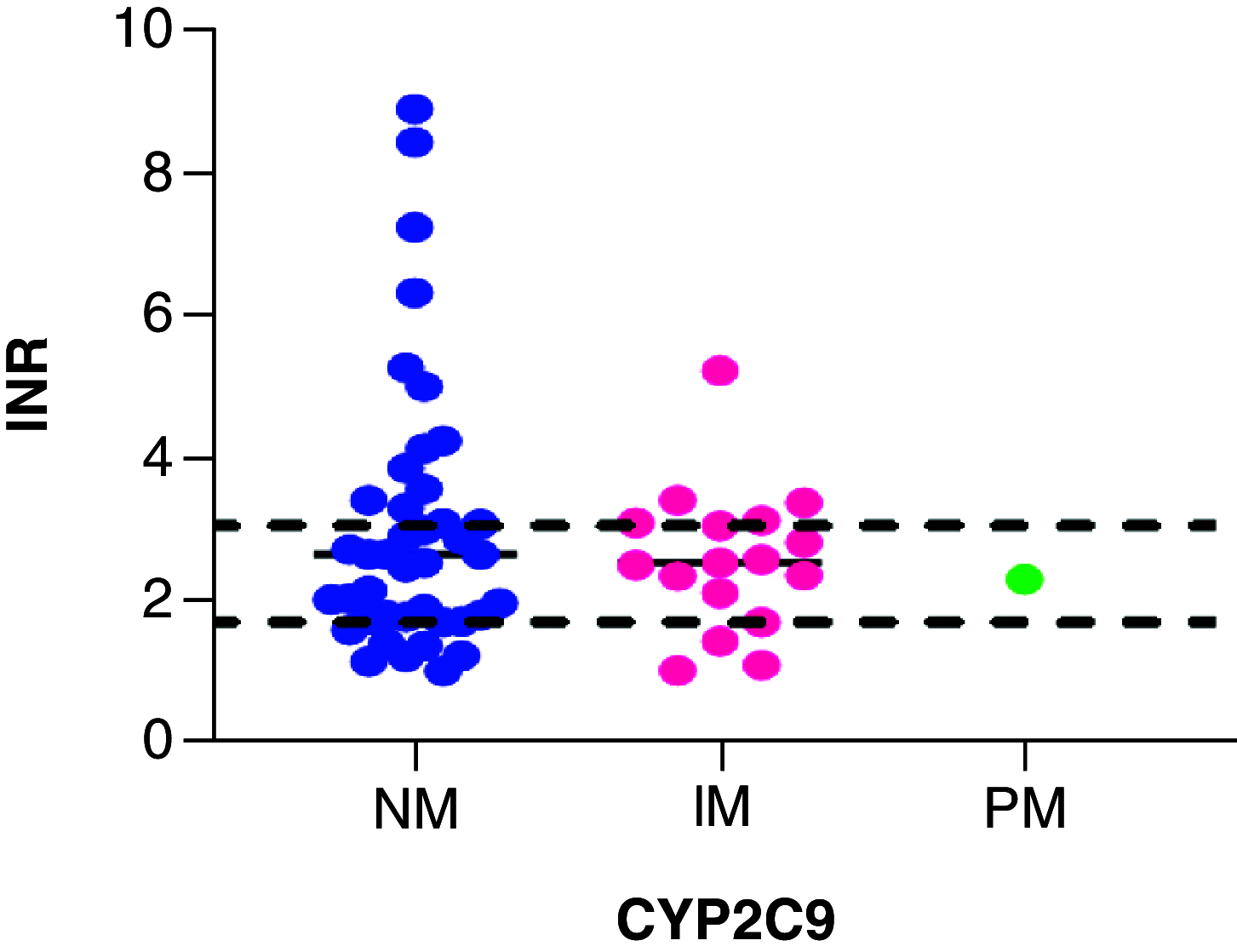
Aim: A prospective observational study was conducted to evaluate the feasibility of implementing clinical guidelines for warfarin dosing in black Zimbabwean patients. Methods: CYP2C9*5, CYP2C9*6, CYP2C9*8 and CYP2C9*11 and VKORC1 c. 1639 G>A variations were observed in 62 study patients. Results & Conclusion: Overall, 39/62 (62.90%) participants did not receive a warfarin starting dose as would have been recommended by Clinical Pharmacogenetics Implementation Consortium guidelines. US FDA and Dutch Pharmacogenetics Working Group guidelines are based on CYP2C9*2 and CYP2C9*3 only, hence, unlikely useful in this cohort, where such variants were not detected. Clinical Pharmacogenetics Implementation Consortium guidelines, on the other hand, have a specific recommendation on the African-specific variants CYP2C9*5, CYP2C9*6 and CYP2C9*11, and are hence suitable for implementation in Zimbabwe and would help optimize warfarin doses in patients in the study cohort.
Keywords: CYP2C9; VKORC1; genetic variation; pharmacogenomics; warfarin.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Sjögren V, Grzymala-Lubanski B, Renlund H, Friberg L, Lip GY, Svensson PJ et al. Safety and efficacy of well managed warfarin. Thromb. Haemost. 113(06), 1370–1377 (2015). - PubMed
-
- Prudden HJ, Achilles SL, Schocken C, Broutet N, Canfell K, Akaba H et al. Understanding the public health value and defining preferred product characteristics for therapeutic human papillomavirus (HPV) vaccines: World Health Organization consultations, October 2021–March 2022. Vaccine 40(41), 5843–5855 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
