Appropriateness of strategy comparisons in cost-effectiveness analyses of infant pneumococcal vaccination: a systematic review
- PMID: 37435736
- PMCID: PMC11570002
- DOI: 10.1017/S0266462323000351
Appropriateness of strategy comparisons in cost-effectiveness analyses of infant pneumococcal vaccination: a systematic review
Abstract
Objectives: Cost-effectiveness analysis (CEA) is the standard framework for informing the efficient allocation of scarce healthcare resources. The importance of considering all relevant intervention strategies and appropriate incremental comparisons have both long been recognized in CEA. Failure to apply methods correctly can lead to suboptimal policies. Our objective is to assess if CEAs of infant pneumococcal vaccination apply appropriate methods with respect to the completeness of strategies assessed and incremental comparisons between them.
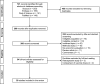
Methods: We conducted a systematic search of the PubMed, Scopus, Embase, and Web of Science databases and performed a comparative analysis of the retrieved pneumococcal vaccination CEAs. We checked the appropriateness of the incremental analyses by attempting to replicate the published incremental cost-effectiveness (CE) ratios from the reported costs and health effects.
Results: Our search returned twenty-nine eligible articles. Most studies failed to recognize one or more intervention strategies (n = 21). Incremental comparisons were questionable in four CEAs and insufficient reporting of cost and health effect estimates was identified in three studies. Overall, we only found four studies that made appropriate comparisons between all strategies. Lastly, study findings appear to be strongly associated with manufacturer sponsorship.
Conclusions: We found considerable scope for improvement regarding strategy comparison in the infant pneumococcal vaccination literature. To prevent overestimation of the CE of new vaccines, we urge greater adherence to existing guidelines recommending that all available strategies are evaluated to capture relevant comparators for CE evaluation. Closer adherence to existing guidelines will generate better evidence, leading to more effective vaccination policies.
Keywords: comparator selection; economic evaluation; health outcomes; pneumococcal disease; vaccines.
Conflict of interest statement
The authors declare none.
Figures

Similar articles
-
Cost-Effectiveness of Treatment Options for Neuropathic Pain: a Systematic Review.Pharmacoeconomics. 2019 May;37(5):669-688. doi: 10.1007/s40273-018-00761-6. Pharmacoeconomics. 2019. PMID: 30637713
-
Impact of vaccine herd-protection effects in cost-effectiveness analyses of childhood vaccinations. A quantitative comparative analysis.PLoS One. 2017 Mar 1;12(3):e0172414. doi: 10.1371/journal.pone.0172414. eCollection 2017. PLoS One. 2017. PMID: 28249046 Free PMC article.
-
Exploring the cost-effectiveness of child dental caries prevention programmes. Are we comparing apples and oranges?Evid Based Dent. 2020 Mar;21(1):5-7. doi: 10.1038/s41432-020-0085-7. Evid Based Dent. 2020. PMID: 32221482
-
Testing strategies for Lynch syndrome in people with endometrial cancer: systematic reviews and economic evaluation.Health Technol Assess. 2021 Jun;25(42):1-216. doi: 10.3310/hta25420. Health Technol Assess. 2021. PMID: 34169821 Free PMC article.
-
Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines.BMJ. 2010 Jun 2;340:c2509. doi: 10.1136/bmj.c2509. BMJ. 2010. PMID: 20519267
References
-
- Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: Burden of disease. Clin Microbiol Infect. 2014;20:45–51. - PubMed
-
- Bogaert D, Hermans PWM, Adrian P V., Rümke HC, De Groot R. Pneumococcal vaccines: An update on current strategies. Vaccine. 2004;22:2209–2220. - PubMed
-
- Principi N, Esposito S. The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on serotype 19A invasive pneumococcal disease. Expert Rev Vaccines. 2015;14:1359–1366. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

