Sweet and sour synergy: exploring the antibacterial and antibiofilm activity of acetic acid and vinegar combined with medical-grade honeys
- PMID: 37435775
- PMCID: PMC10433418
- DOI: 10.1099/mic.0.001351
Sweet and sour synergy: exploring the antibacterial and antibiofilm activity of acetic acid and vinegar combined with medical-grade honeys
Abstract
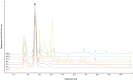
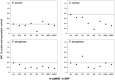
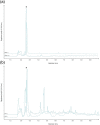
Oxymel, a combination of honey and vinegar, has been used as a remedy for wounds and infections in historical and traditional medical settings. While honey is now clinically used to treat infected wounds, this use of a complex, raw natural product (NP) mixture is unusual in modern western medicine. Research into the antimicrobial activity of NPs more usually focuses on finding a single active compound. The acetic acid in vinegar is known to have antibacterial activity at low concentrations and is in clinical use to treat burn wound infections. Here, we investigated the potential for synergistic activity of different compounds present in a complex ingredient used in historical medicine (vinegar) and in an ingredient mixture (oxymel). We conducted a systematic review to investigate published evidence for antimicrobial effects of vinegars against human pathogenic bacteria and fungi. No published studies have explicitly compared the activity of vinegar with that of a comparable concentration of acetic acid. We then characterized selected vinegars by HPLC and assessed the antibacterial and antibiofilm activity of the vinegars and acetic acid, alone and in combination with medical-grade honeys, against Pseudomonas aeruginosa and Staphylococcus aureus. We found that some vinegars have antibacterial activity that exceeds that predicted by their acetic acid content alone, but that this depends on the bacterial species being investigated and the growth conditions (media type, planktonic vs. biofilm). Pomegranate vinegars may be particularly interesting candidates for further study. We also conclude that there is potential for acetic acid, and some vinegars, to show synergistic antibiofilm activity with manuka honey.
Keywords: antimicrobials; biofilm; natural products; synergy.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Kuropatnicki AK, Kłósek M, Kucharzewski M. Honey as medicine: historical perspectives. J Apic Res. 2018;57:113–118. doi: 10.1080/00218839.2017.1411182. - DOI
-
- Mazza S, Murooka Y. In: Vinegars of the World. Solieri L, Giudici P, editors. Milano: Springer Milan; 2009. Vinegars through the ages; pp. 17–39. - DOI
-
- Krug I. In: Wounds and Wound Repair in Medieval Culture. Krug I, Tracy L, DeVries K, editors. Brill; 2015. The wounded soldier: honey and late medieval military medicine; pp. 194–214. - DOI
-
- Zargaran A, Zarshenas MM, Mehdizadeh A, Mohagheghzadeh A. Oxymel in medieval Persia. Pharm Hist. 2012;42:11–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

