Conformational regulation and target-myristoyl switch of calcineurin B homologous protein 3
- PMID: 37435805
- PMCID: PMC10368425
- DOI: 10.7554/eLife.83868
Conformational regulation and target-myristoyl switch of calcineurin B homologous protein 3
Abstract
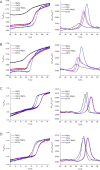
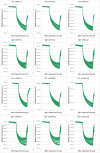
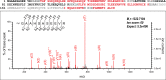
Calcineurin B homologous protein 3 (CHP3) is an EF-hand Ca2+-binding protein involved in regulation of cancerogenesis, cardiac hypertrophy, and neuronal development through interactions with sodium/proton exchangers (NHEs) and signalling proteins. While the importance of Ca2+ binding and myristoylation for CHP3 function has been recognized, the underlying molecular mechanism remained elusive. In this study, we demonstrate that Ca2+ binding and myristoylation independently affect the conformation and functions of human CHP3. Ca2+ binding increased local flexibility and hydrophobicity of CHP3 indicative of an open conformation. The Ca2+-bound CHP3 exhibited a higher affinity for NHE1 and associated stronger with lipid membranes compared to the Mg2+-bound CHP3, which adopted a closed conformation. Myristoylation enhanced the local flexibility of CHP3 and decreased its affinity to NHE1 independently of the bound ion, but did not affect its binding to lipid membranes. The data exclude the proposed Ca2+-myristoyl switch for CHP3. Instead, a Ca2+-independent exposure of the myristoyl moiety is induced by binding of the target peptide to CHP3 enhancing its association to lipid membranes. We name this novel regulatory mechanism 'target-myristoyl switch'. Collectively, the interplay of Ca2+ binding, myristoylation, and target binding allows for a context-specific regulation of CHP3 functions.
Keywords: CHP3; Ca2+-binding proteins; NHE1; biochemistry; chemical biology; conformational changes; human; myristoyl switch; myristoylation; tescalcin.
© 2023, Becker, Fuchs et al.
Conflict of interest statement
FB, SF, LR, FD, WB, SL, JH, SH, CK, BW, EM, CH No competing interests declared, US is an employee and shareholder of Logopharm GmbH that produces ComplexioLyte 47 used in this study, BF is shareholder of Logopharm GmbH. The company provides ComplexioLyte reagents to academic institutions on a non-profit basis
Figures










Update of
- doi: 10.1101/2022.09.23.509142
Similar articles
-
N-myristoylation and Ca2+ binding of calcineurin B homologous protein CHP3 are required to enhance Na+/H+ exchanger NHE1 half-life and activity at the plasma membrane.J Biol Chem. 2012 Oct 26;287(44):36883-95. doi: 10.1074/jbc.M112.394700. Epub 2012 Sep 14. J Biol Chem. 2012. PMID: 22984264 Free PMC article.
-
Calcineurin B homologous protein 3 promotes the biosynthetic maturation, cell surface stability, and optimal transport of the Na+/H+ exchanger NHE1 isoform.J Biol Chem. 2008 May 2;283(18):12456-67. doi: 10.1074/jbc.M800267200. Epub 2008 Mar 5. J Biol Chem. 2008. PMID: 18321853 Free PMC article.
-
Calcineurin B homologous protein 3 binds with high affinity to the CHP binding domain of the human sodium/proton exchanger NHE1.Sci Rep. 2018 Oct 4;8(1):14837. doi: 10.1038/s41598-018-33096-5. Sci Rep. 2018. PMID: 30287853 Free PMC article.
-
Emerging roles of the single EF-hand Ca2+ sensor tescalcin in the regulation of gene expression, cell growth and differentiation.J Cell Sci. 2016 Oct 1;129(19):3533-3540. doi: 10.1242/jcs.191486. Epub 2016 Sep 8. J Cell Sci. 2016. PMID: 27609838 Free PMC article. Review.
-
Calcineurin homologous protein: a multifunctional Ca2+-binding protein family.Am J Physiol Renal Physiol. 2012 Jul 15;303(2):F165-79. doi: 10.1152/ajprenal.00628.2011. Epub 2011 Dec 21. Am J Physiol Renal Physiol. 2012. PMID: 22189947 Free PMC article. Review.
References
-
- Andrade J, Zhao H, Titus B, Timm Pearce S, Barroso M. The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements. Molecular Biology of the Cell. 2004;15:481–496. doi: 10.1091/mbc.e03-07-0500. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

