Mechanistic Investigation of tert-Butanol's Impact on Biopharmaceutical Formulations: When Experiments Meet Molecular Dynamics
- PMID: 37435823
- PMCID: PMC10410665
- DOI: 10.1021/acs.molpharmaceut.3c00125
Mechanistic Investigation of tert-Butanol's Impact on Biopharmaceutical Formulations: When Experiments Meet Molecular Dynamics
Abstract
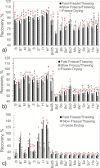
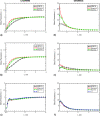
The use of tert-butyl alcohol for the lyophilization of pharmaceuticals has seen an uptick over the past years. Its advantages include increased solubility of hydrophobic drugs, enhanced product stability, shorter reconstitution time, and decreased processing time. While the mechanisms of protein stabilization exerted by cryo- and lyo-protectants are well known when water is the solvent of choice, little is known for organic solvents. This work investigates the interactions between two model proteins, namely, lactate dehydrogenase and myoglobin, and various excipients (mannitol, sucrose, 2-hydroxypropyl-β-cyclodextrin and Tween 80) in the presence of tert-butyl alcohol. We thermally characterized mixtures of these components by differential scanning calorimetry and freeze-drying microscopy. We also spectroscopically evaluated the protein recovery after freezing and freeze-drying. We additionally performed molecular dynamics simulations to elucidate the interactions in ternary mixtures of the herein-investigated excipients, tert-butyl alcohol and the proteins. Both experiments and simulations revealed that tert-butyl alcohol had a detrimental impact on the recovery of the two investigated proteins, and no combination of excipients yielded a satisfactory recovery when the organic solvent was present within the formulation. Simulations suggested that the denaturing effect of tert-butyl alcohol was related to its propensity to accumulate in the proximity of the peptide surface, especially near positively charged residues.
Keywords: cosolvent formulations; cyclodextrins; freeze-drying; molecular dynamics; protein stability; tert-butyl alcohol.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Freeze-drying of tert-butyl alcohol/water cosolvent systems: effects of formulation and process variables on residual solvents.J Pharm Sci. 1998 Apr;87(4):491-5. doi: 10.1021/js9702832. J Pharm Sci. 1998. PMID: 9548903
-
[Factors influencing the content of residual tert-butyl alcohol in cyclodextrin complex prepared by lyophilization cosolvent system].Yao Xue Xue Bao. 2007 Mar;42(3):314-7. Yao Xue Xue Bao. 2007. PMID: 17520833 Chinese.
-
Freeze-Drying From Organic Cosolvent Systems, Part 1: Thermal Analysis of Cosolvent-Based Placebo Formulations in the Frozen State.J Pharm Sci. 2018 Mar;107(3):887-896. doi: 10.1016/j.xphs.2017.11.003. Epub 2017 Nov 10. J Pharm Sci. 2018. PMID: 29133233
-
Mannitol as an Excipient for Lyophilized Injectable Formulations.J Pharm Sci. 2023 Jan;112(1):19-35. doi: 10.1016/j.xphs.2022.08.029. Epub 2022 Aug 27. J Pharm Sci. 2023. PMID: 36030846 Review.
-
Factors Influencing the Retention of Organic Solvents in Products Freeze-Dried From Co-Solvent Systems.J Pharm Sci. 2018 Aug;107(8):2005-2012. doi: 10.1016/j.xphs.2018.04.001. Epub 2018 Apr 9. J Pharm Sci. 2018. PMID: 29649470 Review.
Cited by
-
Predictive Model Building for Aggregation Kinetics Based on Molecular Dynamics Simulations of an Antibody Fragment.Mol Pharm. 2024 Nov 4;21(11):5827-5841. doi: 10.1021/acs.molpharmaceut.4c00859. Epub 2024 Sep 30. Mol Pharm. 2024. PMID: 39348223 Free PMC article.
-
Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges.Pharmaceuticals (Basel). 2025 Jun 17;18(6):908. doi: 10.3390/ph18060908. Pharmaceuticals (Basel). 2025. PMID: 40573303 Free PMC article. Review.
-
Molecular Dynamics Simulations Reveal How Competing Protein-Surface Interactions for Glycine, Citrate, and Water Modulate Stability in Antibody Fragment Formulations.Mol Pharm. 2024 Nov 4;21(11):5497-5509. doi: 10.1021/acs.molpharmaceut.4c00332. Epub 2024 Oct 21. Mol Pharm. 2024. PMID: 39431440 Free PMC article.
References
-
- Franks F.; Auffret T.. Freeze-Drying of Pharmaceuticals and Biopharmaceuticals; The Royal Society of Chemistry, 2007.
-
- Wang W.; Roberts C. J.. Aggregation of Therapeutic Proteins, 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

