Ru-NHC-Catalyzed Asymmetric, Complete Hydrogenation of Indoles and Benzofurans: One Catalyst with Dual Function
- PMID: 37435957
- PMCID: PMC10375535
- DOI: 10.1021/jacs.3c04983
Ru-NHC-Catalyzed Asymmetric, Complete Hydrogenation of Indoles and Benzofurans: One Catalyst with Dual Function
Abstract
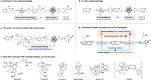
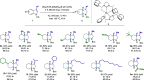
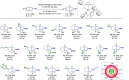
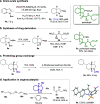
The highly enantioselective and complete hydrogenation of protected indoles and benzofurans has been developed, affording facile access to a range of chiral three-dimensional octahydroindoles and octahydrobenzofurans, which are prevalent in many bioactive molecules and organocatalysts. Remarkably, we are in control of the nature of the ruthenium N-heterocyclic carbene complex and employed the complex as both homogeneous and heterogeneous catalysts, providing new avenues for its potential applications in the asymmetric hydrogenation of more challenging aromatic compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Enantio- and Diastereoselective, Complete Hydrogenation of Benzofurans by Cascade Catalysis.Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13677-13681. doi: 10.1002/anie.202103910. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33844391 Free PMC article.
-
Ru-NHC-Catalyzed Asymmetric Hydrogenation of 2-Quinolones to Chiral 3,4-Dihydro-2-Quinolones.Angew Chem Int Ed Engl. 2021 Oct 18;60(43):23193-23196. doi: 10.1002/anie.202108503. Epub 2021 Sep 22. Angew Chem Int Ed Engl. 2021. PMID: 34460127 Free PMC article.
-
Consecutive Intermolecular Reductive Amination/Asymmetric Hydrogenation: Facile Access to Sterically Tunable Chiral Vicinal Diamines and N-Heterocyclic Carbenes.Angew Chem Int Ed Engl. 2019 Nov 18;58(47):16831-16834. doi: 10.1002/anie.201909919. Epub 2019 Oct 11. Angew Chem Int Ed Engl. 2019. PMID: 31486574
-
Homogeneous palladium-catalyzed asymmetric hydrogenation.Chem Soc Rev. 2013 Jan 21;42(2):497-511. doi: 10.1039/c2cs35333d. Chem Soc Rev. 2013. PMID: 23138972 Review.
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
Cited by
-
Cobalt-catalyzed conformationally restricted alkylarylation enables divergent access to Csp3-rich N-heterocycles.Chem Sci. 2024 Sep 3;15(39):16250-8. doi: 10.1039/d4sc04056b. Online ahead of print. Chem Sci. 2024. PMID: 39297003 Free PMC article.
-
Synthesis of Chiral δ-Aminoboronic Esters by Enantioselective Hydrogenation of 1,2-Azaborines.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202504419. doi: 10.1002/anie.202504419. Epub 2025 Apr 17. Angew Chem Int Ed Engl. 2025. PMID: 40192605
-
Access to C(sp3) borylated and silylated cyclic molecules: hydrogenation of corresponding arenes and heteroarenes.RSC Adv. 2024 Apr 2;14(15):10590-10607. doi: 10.1039/d4ra00491d. eCollection 2024 Mar 26. RSC Adv. 2024. PMID: 38567346 Free PMC article.
-
Improving reproducibility through condition-based sensitivity assessments: application, advancement and prospect.Chem Sci. 2024 Aug 30;15(36):14548-55. doi: 10.1039/d4sc03017f. Online ahead of print. Chem Sci. 2024. PMID: 39263664 Free PMC article. Review.
References
-
- Mortier J., Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; John Wiley & Sons, 2015.
LinkOut - more resources
Full Text Sources

