Hepatitis delta virus RNA decline post-inoculation in human NTCP transgenic mice is biphasic
- PMID: 37436080
- PMCID: PMC10470517
- DOI: 10.1128/mbio.01008-23
Hepatitis delta virus RNA decline post-inoculation in human NTCP transgenic mice is biphasic
Abstract
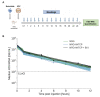
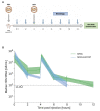
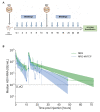
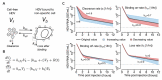
Chronic infection with hepatitis B and delta viruses (HDV) is the most serious form of viral hepatitis due to more severe manifestations of an accelerated progression to liver fibrosis, cirrhosis, and hepatocellular carcinoma. We characterized early HDV kinetics post-inoculation and incorporated mathematical modeling to provide insights into host-HDV dynamics. We analyzed HDV RNA serum viremia in 192 immunocompetent (C57BL/6) and immunodeficient (NRG) mice that did or did not transgenically express the HDV receptor-human sodium taurocholate co-transporting polypeptide (hNTCP). Kinetic analysis indicates an unanticipated biphasic decline consisting of a sharp first-phase and slower second-phase decline regardless of immunocompetence. HDV decline after re-inoculation again followed a biphasic decline; however, a steeper second-phase HDV decline was observed in NRG-hNTCP mice compared to NRG mice. HDV-entry inhibitor bulevirtide administration and HDV re-inoculation indicated that viral entry and receptor saturation are not major contributors to clearance, respectively. The biphasic kinetics can be mathematically modeled by assuming the existence of a non-specific-binding compartment with a constant on/off-rate and the steeper second-phase decline by a loss of bound virus that cannot be returned as free virus to circulation. The model predicts that free HDV is cleared with a half-life of 35 minutes (standard error, SE: 6.3), binds to non-specific cells with a rate of 0.05 per hour (SE: 0.01), and returns as free virus with a rate of 0.11 per hour (SE: 0.02). Characterizing early HDV-host kinetics elucidates how quickly HDV is either cleared or bound depending on the immunological background and hNTCP presence. IMPORTANCE The persistence phase of HDV infection has been studied in some animal models; however, the early kinetics of HDV in vivo is incompletely understood. In this study, we characterize an unexpectedly HDV biphasic decline post-inoculation in immunocompetent and immunodeficient mouse models and use mathematical modeling to provide insights into HDV-host dynamics.
Keywords: animal model; hepatitis B virus; hepatitis delta virus; mathematical modeling; viral hepatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Hepatitis delta virus RNA decline post inoculation in human NTCP transgenic mice is biphasic.bioRxiv [Preprint]. 2023 Feb 17:2023.02.17.528964. doi: 10.1101/2023.02.17.528964. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0100823. doi: 10.1128/mbio.01008-23. PMID: 36824865 Free PMC article. Updated. Preprint.
References
-
- Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Fälth M, Stindt J, Königer C, Nassal M, Kubitz R, Sültmann H, Urban S. 2014. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146:1070–1083. doi:10.1053/j.gastro.2013.12.024 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

