Bowel Urgency in Ulcerative Colitis: Current Perspectives and Future Directions
- PMID: 37436151
- PMCID: PMC10617668
- DOI: 10.14309/ajg.0000000000002404
Bowel Urgency in Ulcerative Colitis: Current Perspectives and Future Directions
Abstract
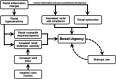
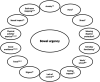
Bowel urgency (BU), the sudden or immediate need for a bowel movement, is one of the most common and disruptive symptoms experienced by patients with ulcerative colitis (UC). Distinct from the separate symptom of increased stool frequency, BU has a substantial negative impact on quality of life and psychosocial functioning. Among patients with UC, BU is one of the top reasons for treatment dissatisfaction and one of the symptoms patients most want improved. Patients may not discuss BU often due to embarrassment, and healthcare providers may not address the symptom adequately due to the lack of awareness of validated tools and/or knowledge of the importance of assessing BU. The mechanism of BU in UC is multifactorial and includes inflammatory changes in the rectum that may be linked to hypersensitivity and reduced compliance of the rectum. Responsive and reliable patient-reported outcome measures of BU are needed to provide evidence of treatment benefits in clinical trials and facilitate communication in clinical practice. This review discusses the pathophysiology and clinical importance of BU in UC and its impact on the quality of life and psychosocial functioning. Patient-reported outcome measures developed to assess the severity of BU in UC are discussed alongside overviews of treatment options and clinical guidelines. Implications for the future management of UC from the perspective of BU are also explored.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- US Department of Health and Human Services (FDA Guidance for UC Endpoints). 2016. (https://www.fda.gov/media/99526/download). Accessed October 26, 2022.
-
- MedlinePlus (https://medlineplus.gov/ency/article/003131.htm). Accessed October 26, 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

