Tuberculosis in children and adolescents with rheumatic diseases using biologic agents: an integrative review
- PMID: 37436237
- PMCID: PMC10332438
- DOI: 10.1590/1984-0462/2024/42/2022084
Tuberculosis in children and adolescents with rheumatic diseases using biologic agents: an integrative review
Abstract
Objective: To conduct a bibliographic review on tuberculosis (TB) disease in children and adolescents with rheumatic diseases, being managed with biologic therapy.
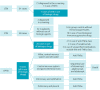
Data source: An integrative review with a search in the U.S. National Library of Medicine and the National Institutes of Health (PubMed) using the following descriptors and Boolean operators: (["tuberculosis"] AND (["children"] OR ["adolescent"]) AND ["rheumatic diseases"] AND (["tumor necrosis factor-alpha"] OR ["etanercept"] OR ["adalimumab"] OR ["infliximab"] OR ["biological drugs"] OR ["rituximab"] OR ["belimumab"] OR ["tocilizumab"] OR ["canakinumab"] OR ["golimumab"] OR ["secukinumab"] OR ["ustekinumab"] OR ["tofacitinib"] OR ["baricitinib"] OR ["anakinra"] OR ["rilonacept"] OR ["abatacept"]), between January 2010 and October 2021.
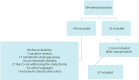
Data synthesis: Thirty-seven articles were included, with the total number of 36,198 patients. There were 81 cases of latent tuberculosis infection (LTBI), 80 cases of pulmonary tuberculosis (PTB), and four of extrapulmonary tuberculosis (EPTB). The main rheumatic disease was juvenile idiopathic arthritis. Among LTBI cases, most were diagnosed at screening and none progressed to TB disease during follow-up. Of the TB cases using biologics, most used tumor necrosis factor-alpha inhibitors (anti-TNFα) drugs. There was only one death.
Conclusions: The study revealed a low rate of active TB in pediatric patients using biologic therapy. Screening for LTBI before initiating biologics should be done in all patients, and treatment, in cases of positive screening, plays a critical role in preventing progression to TB disease.
Objetivo:: Fazer um levantamento bibliográfico referente à tuberculose (TB) em crianças e adolescentes com doenças reumáticas, em uso de imunobiológicos.
Fonte de dados:: Revisão integrativa com busca na base United States National Library of Medicine (PubMed) utilizando os descritores e operadores booleanos: ([“tuberculosis”] AND ([“children”] OR [“adolescent”]) AND [“rheumatic diseases”] AND ([“tumor necrosis fator-alpha”] OR [“etanercept”] OR [“adalimumab”] OR [“infliximab”] OR [“biological drugs”] OR [“rituximab”] OR [“belimumab”] OR [“tocilizumab”] OR [“canakinumab”] OR [“golimumab”] OR [“secukinumab”] OR [“ustekinumab”] OR [“tofacitinib”] OR [“baricitinib”] OR [“anakinra”] OR [“rilonacept”] OR [“abatacept”]), entre janeiro de 2010 e outubro de 2021.
Síntese de dados:: Trinta e sete artigos foram incluídos, com o total de 36.198 pacientes. Houve 81 casos de tuberculose latente (ILTB), 80 casos de tuberculose pulmonar (TBP) e quatro casos de tuberculose extrapulmonar (TBEP). A principal doença reumática foi a artrite idiopática juvenil. Entre os casos de ILTB, a maioria foi diagnosticada no rastreio e nenhum evoluiu para a TB. Dos casos de TB em uso de imunobiológicos, a maioria utilizava fármacos antiTNFα. Houve somente um caso de óbito.
Conclusões:: O estudo demonstrou baixa taxa de TB nos pacientes pediátricos em uso de imunobiológicos. O rastreio para ILTB antes do início da terapia com agentes biológicos deve ser realizado em todos os pacientes, e o tratamento, nos casos de rastreio positivo, é importante para evitar a progressão para TB doença.
Conflict of interest statement
Figures
Similar articles
-
Biologic therapy for inflammatory arthritis and latent tuberculosis: real world experience from a high prevalence area in the United Kingdom.Clin Rheumatol. 2015 Dec;34(12):2141-5. doi: 10.1007/s10067-015-3099-3. Epub 2015 Oct 24. Clin Rheumatol. 2015. PMID: 26497501
-
Balancing Effective Treatments With Potential Threats: The Impact of Biologic Agent Use on Tuberculosis Development in Children With Chronic Inflammatory Disorders.Int J Rheum Dis. 2025 Feb;28(2):e70145. doi: 10.1111/1756-185X.70145. Int J Rheum Dis. 2025. PMID: 39989306
-
Tuberculosis in biologic users for rheumatic diseases: results from the South African Biologics Registry (SABIO).Ann Rheum Dis. 2020 Feb;79(2):292-299. doi: 10.1136/annrheumdis-2019-216128. Epub 2019 Dec 2. Ann Rheum Dis. 2020. PMID: 31791950
-
Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics.Expert Rev Clin Immunol. 2020 Feb;16(2):207-228. doi: 10.1080/1744666X.2019.1705785. Epub 2020 Jan 11. Expert Rev Clin Immunol. 2020. PMID: 31852268 Review.
-
Risk of tuberculosis reactivation associated with traditional disease modifying anti-rheumatic drugs and non-anti-tumor necrosis factor biologics in patients with rheumatic disorders and suggestion for clinical practice.Expert Opin Drug Saf. 2019 May;18(5):415-425. doi: 10.1080/14740338.2019.1612872. Epub 2019 May 8. Expert Opin Drug Saf. 2019. PMID: 31066297 Review.
Cited by
-
Osteoarticular tuberculosis: imaging findings in pediatric patients.Pediatr Radiol. 2025 Jan;55(1):104-114. doi: 10.1007/s00247-024-06092-3. Epub 2024 Nov 16. Pediatr Radiol. 2025. PMID: 39549102 Review.
-
Tuberculosis in children and adolescents using biological agents: a nationwide cohort study from Turkey.BMC Pulm Med. 2025 Apr 25;25(1):196. doi: 10.1186/s12890-025-03616-x. BMC Pulm Med. 2025. PMID: 40281511 Free PMC article.
-
Tuberculosis among children and adolescents with rheumatic diseases - case series.Pediatr Rheumatol Online J. 2023 Nov 10;21(1):136. doi: 10.1186/s12969-023-00918-4. Pediatr Rheumatol Online J. 2023. PMID: 37950309 Free PMC article.
-
Post-marketing safety of anakinra and canakinumab: a real-world pharmacovigilance study based on FDA adverse event reporting system.Front Pharmacol. 2025 Apr 30;16:1483669. doi: 10.3389/fphar.2025.1483669. eCollection 2025. Front Pharmacol. 2025. PMID: 40371323 Free PMC article.
References
-
- World Health Organization [homepage on the Internet] Global tuberculosis report 2020. Geneva: World Health Organization; 2020. [cited 2022 Mar. 03]. Available from: https://www.who.int/publications/i/item/9789240013131 .
-
- Leite JC, Junior, Ramos RT, Robazzi TC. Tratamento da tuberculose latente em pacientes com doenças reumáticas juvenis: uma revisão sistemática. Rev Bras Reumatol. 2017;57:245–53. doi: 10.1016/j.rbr.2016.11.005. - DOI
-
- Sevcic K, Orban I, Brodszky V, Bazso A, Balogh Z, Poor G, et al. Experiences with tumour necrosis factor-{alpha} inhibitors in patients with juvenile idiopathic arthritis: Hungarian data from the National Institute of Rheumatology and Physiotherapy Registry. Rheumatology (Oxford). 2011;50:1337–40. doi: 10.1093/rheumatology/ker103. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical