Effect of Nasal Continuous Positive Airway Pressure vs Heated Humidified High-Flow Nasal Cannula on Feeding Intolerance in Preterm Infants With Respiratory Distress Syndrome: The ENTARES Randomized Clinical Trial
- PMID: 37436750
- PMCID: PMC10339152
- DOI: 10.1001/jamanetworkopen.2023.23052
Effect of Nasal Continuous Positive Airway Pressure vs Heated Humidified High-Flow Nasal Cannula on Feeding Intolerance in Preterm Infants With Respiratory Distress Syndrome: The ENTARES Randomized Clinical Trial
Abstract
Importance: Respiratory distress syndrome and feeding intolerance are common conditions that are often associated with preterm infants. Showing similar efficacy, nasal continuous positive airway pressure (NCPAP) and heated humidified high-flow nasal cannula (HHHFNC) are the most widespread noninvasive respiratory support (NRS) in neonatal intensive care units, but their effect on feeding intolerance is unknown.
Objective: To evaluate the effect of NCPAP vs HHHFNC on high-risk preterm infants with respiratory distress syndrome.
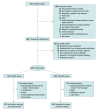
Design, setting, and participants: This multicenter randomized clinical trial involved infants who were born in 1 of 13 neonatal intensive care units in Italy between November 1, 2018, and June 30, 2021. Preterm infants with a gestational age of 25 to 29 weeks, who were suitable for enteral feeding and who proved to be medically stable on NRS for at least 48 hours were enrolled in the study within the first week of life and randomized to receive either NCPAP or HHHFNC. Statistical analysis was performed according to the intention-to-treat approach.
Intervention: NCPAP or HHHFNC.
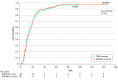
Main outcomes and measures: The primary outcome was the time to full enteral feeding (FEF), defined as an enteral intake of 150 mL/kg per day. Secondary outcomes were the median daily increment of enteral feeding, signs of feeding intolerance, effectiveness of the assigned NRS, peripheral oxygen saturation (SpO2)-fraction of inspired oxygen (FIO2) ratio at changes of NRS, and growth.
Results: Two-hundred forty-seven infants (median [IQR] gestational age, 28 [27-29] weeks; 130 girls [52.6%]) were randomized to the NCPAP group (n = 122) or the HHHFNC group (n = 125). There were no differences in the primary and secondary nutritional outcomes between the 2 groups. The median time to reach FEF was 14 days (95% CI, 11-15 days) in the NCPAP group and 14 days (95% CI, 12-18 days) in the HHHFNC group, and similar results were observed in the subgroup of infants with less than 28 weeks' gestation. On the first NRS change, higher SpO2-FIO2 ratio (median [IQR], 4.6 [4.1-4.7] vs 3.7 [3.2-4.0]; P < .001) and lower rate of ineffectiveness (1 [4.8%] vs 17 [73.9%]; P < .001) were observed in the NCPAP vs HHHFNC group.
Conclusions and relevance: This randomized clinical trial found that NCPAP and HHHFNC had similar effects on feeding intolerance, despite different working mechanisms. Clinicians may tailor respiratory care by selecting and switching between the 2 NRS techniques on the basis of respiratory effectiveness and patient compliance, without affecting feeding intolerance.
Trial registration: ClinicalTrials.gov Identifier: NCT03548324.
Conflict of interest statement
Figures