Regulation of chromatin microphase separation by binding of protein complexes
- PMID: 37436818
- PMCID: PMC10338032
- DOI: 10.7554/eLife.82983
Regulation of chromatin microphase separation by binding of protein complexes
Abstract
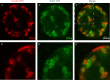
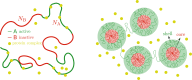
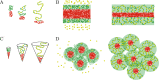
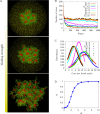
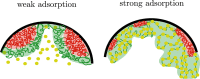
We show evidence of the association of RNA polymerase II (RNAP) with chromatin in a core-shell organization, reminiscent of microphase separation where the cores comprise dense chromatin and the shell, RNAP and chromatin with low density. These observations motivate our physical model for the regulation of core-shell chromatin organization. Here, we model chromatin as a multiblock copolymer, comprising active and inactive regions (blocks) that are both in poor solvent and tend to be condensed in the absence of binding proteins. However, we show that the solvent quality for the active regions of chromatin can be regulated by the binding of protein complexes (e.g., RNAP and transcription factors). Using the theory of polymer brushes, we find that such binding leads to swelling of the active chromatin regions which in turn modifies the spatial organization of the inactive regions. In addition, we use simulations to study spherical chromatin micelles, whose cores comprise inactive regions and shells comprise active regions and bound protein complexes. In spherical micelles the swelling increases the number of inactive cores and controls their size. Thus, genetic modifications affecting the binding strength of chromatin-binding protein complexes may modulate the solvent quality experienced by chromatin and regulate the physical organization of the genome.
Keywords: D. melanogaster; active and inactive chromatin; chromatin organization; chromatin-binding protein complexes; chromosomes; gene expression; microphase separation; physics of living systems.
© 2023, Adame-Arana et al.
Conflict of interest statement
OA, GB, DL, TV, SS No competing interests declared
Figures

Update of
- doi: 10.1101/2022.09.29.510124
Similar articles
-
Association of human TFIID-promoter complexes with silenced mitotic chromatin in vivo.Nat Cell Biol. 2002 Jan;4(1):79-82. doi: 10.1038/ncb733. Nat Cell Biol. 2002. PMID: 11744923
-
Multiblock copolymer solutions in contact with a surface: self-assembly, adsorption, and percolation.Langmuir. 2014 Oct 21;30(41):12400-10. doi: 10.1021/la502945k. Epub 2014 Oct 6. Langmuir. 2014. PMID: 25285477
-
A polymer model for the structural organization of chromatin loops and minibands in interphase chromosomes.Mol Biol Cell. 1998 Nov;9(11):3031-40. doi: 10.1091/mbc.9.11.3031. Mol Biol Cell. 1998. PMID: 9802894 Free PMC article.
-
Nuclear organization of RNA polymerase II transcription.Biochem Cell Biol. 2013 Feb;91(1):22-30. doi: 10.1139/bcb-2012-0059. Epub 2013 Feb 5. Biochem Cell Biol. 2013. PMID: 23442138 Review.
-
Mechanisms and Functions of Chromosome Compartmentalization.Trends Biochem Sci. 2020 May;45(5):385-396. doi: 10.1016/j.tibs.2020.01.002. Epub 2020 Feb 18. Trends Biochem Sci. 2020. PMID: 32311333 Free PMC article. Review.
Cited by
-
Interplay of chromatin organization and mechanics of the cell nucleus.Biophys J. 2024 Oct 1;123(19):3386-3396. doi: 10.1016/j.bpj.2024.08.003. Epub 2024 Aug 8. Biophys J. 2024. PMID: 39126157
-
Nuclear RNA: a transcription-dependent regulator of chromatin structure.Biochem Soc Trans. 2024 Aug 28;52(4):1605-1615. doi: 10.1042/BST20230787. Biochem Soc Trans. 2024. PMID: 39082979 Free PMC article. Review.
-
Active transcription and epigenetic reactions synergistically regulate meso-scale genomic organization.Nat Commun. 2024 May 21;15(1):4338. doi: 10.1038/s41467-024-48698-z. Nat Commun. 2024. PMID: 38773126 Free PMC article.
-
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction.bioRxiv [Preprint]. 2025 Jul 27:2025.07.24.666416. doi: 10.1101/2025.07.24.666416. bioRxiv. 2025. PMID: 40777249 Free PMC article. Preprint.
-
Chromatin conformation, gene transcription, and nucleosome remodeling as an emergent system.Sci Adv. 2025 Jan 10;11(2):eadq6652. doi: 10.1126/sciadv.adq6652. Epub 2025 Jan 10. Sci Adv. 2025. PMID: 39792661 Free PMC article.
References
-
- Adame-Arana O, Safran SA. Confined polymers in a poor solvent: the role of bonding to the surface. Macromolecules. 2021;54:4760–4768. doi: 10.1021/acs.macromol.1c00343. - DOI
-
- Alexander S. Adsorption of chain molecules with a polar head a scaling description. Journal de Physique. 1977;38:983–987. doi: 10.1051/jphys:01977003808098300. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

