A monolayer crystalline covalent network of polyoxometalate clusters
- PMID: 37436995
- PMCID: PMC10337924
- DOI: 10.1126/sciadv.adi6595
A monolayer crystalline covalent network of polyoxometalate clusters
Abstract
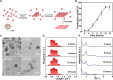
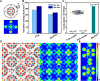
Monolayer two-dimensional (2D) materials are of great interest because of their unique electronic structures, noticeable in-plane confinement effect, and exceptional catalytic properties. Here, we prepared 2D covalent networks of polyoxometalate clusters (CN-POM) featuring monolayer crystalline molecular sheets, formed by the covalent connection between tetragonally arranged POM clusters. The CN-POM shows a superior catalytic efficiency in the oxidation of benzyl alcohol, and the conversion rate is five times higher than that of the POM cluster units. Theoretical calculations show that in-plane electron delocalization of CN-POM contributes to easier electron transfer and increases catalytic efficiency. Moreover, the conductivity of the covalently interconnected molecular sheets was 46 times greater than that of individual POM clusters. The preparation of monolayer covalent network of POM clusters provides a strategy to synthesize advanced cluster-based 2D materials and a precise molecular model to investigate the electronic structure of crystalline covalent networks.
Figures



Similar articles
-
Confining and Highly Dispersing Single Polyoxometalate Clusters in Covalent Organic Frameworks by Covalent Linkages for CO2 Photoreduction.J Am Chem Soc. 2022 Feb 2;144(4):1861-1871. doi: 10.1021/jacs.1c11987. Epub 2022 Jan 20. J Am Chem Soc. 2022. PMID: 35050618
-
Construct Polyoxometalate Frameworks through Covalent Bonds.Inorg Chem. 2015 Sep 8;54(17):8699-704. doi: 10.1021/acs.inorgchem.5b01326. Epub 2015 Aug 19. Inorg Chem. 2015. PMID: 26286321
-
Precise Assembly of Polyoxometalates at Single-cluster Levels.Angew Chem Int Ed Engl. 2023 Mar 6;62(11):e202217764. doi: 10.1002/anie.202217764. Epub 2023 Jan 18. Angew Chem Int Ed Engl. 2023. PMID: 36577699 Review.
-
Atomically Precise Ternary Cluster: Polyoxometalate Cluster Sandwiched by Gold Clusters Protected by N-Heterocyclic Carbenes.Angew Chem Int Ed Engl. 2024 Nov 25;63(48):e202408310. doi: 10.1002/anie.202408310. Epub 2024 Oct 24. Angew Chem Int Ed Engl. 2024. PMID: 39210521
-
Fabricating sub-nanometer materials through cluster assembly.Chem Sci. 2022 Sep 26;13(42):12280-12289. doi: 10.1039/d2sc03813g. eCollection 2022 Nov 2. Chem Sci. 2022. PMID: 36382289 Free PMC article. Review.
Cited by
-
Single-layer cluster ionic-chain networks with tetragonal pores.Nat Commun. 2025 Jul 1;16(1):5778. doi: 10.1038/s41467-025-60879-y. Nat Commun. 2025. PMID: 40592907 Free PMC article.
-
Modular assembly of polyoxometalate clusters at the sub-1 nm scale.Nat Protoc. 2025 Jul 22. doi: 10.1038/s41596-025-01212-1. Online ahead of print. Nat Protoc. 2025. PMID: 40696073 Review.
References
-
- Wang Q. H., Kalantar-Zadeh K., Kis A., Coleman J. N., Strano M. S., Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012). - PubMed
-
- Low T., Chaves A., Caldwell J. D., Kumar A., Fang N. X., Avouris P., Heinz T. F., Guinea F., Martin-Moreno L., Koppens F., Polaritons in layered two-dimensional materials. Nat. Mater. 16, 182–194 (2017). - PubMed
-
- Zhou Y., Yin H., Ai S., Applications of two-dimensional layered nanomaterials in photoelectrochemical sensors: A comprehensive review. Chem. Soc. Rev. 447, 214156 (2021).
LinkOut - more resources
Full Text Sources

