Vaccination with Plasmodium vivax Duffy-binding protein inhibits parasite growth during controlled human malaria infection
- PMID: 37437014
- PMCID: PMC7615121
- DOI: 10.1126/scitranslmed.adf1782
Vaccination with Plasmodium vivax Duffy-binding protein inhibits parasite growth during controlled human malaria infection
Abstract
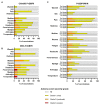
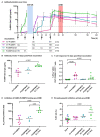
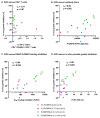
There are no licensed vaccines against Plasmodium vivax. We conducted two phase 1/2a clinical trials to assess two vaccines targeting P. vivax Duffy-binding protein region II (PvDBPII). Recombinant viral vaccines using chimpanzee adenovirus 63 (ChAd63) and modified vaccinia virus Ankara (MVA) vectors as well as a protein and adjuvant formulation (PvDBPII/Matrix-M) were tested in both a standard and a delayed dosing regimen. Volunteers underwent controlled human malaria infection (CHMI) after their last vaccination, alongside unvaccinated controls. Efficacy was assessed by comparisons of parasite multiplication rates in the blood. PvDBPII/Matrix-M, given in a delayed dosing regimen, elicited the highest antibody responses and reduced the mean parasite multiplication rate after CHMI by 51% (n = 6) compared with unvaccinated controls (n = 13), whereas no other vaccine or regimen affected parasite growth. Both viral-vectored and protein vaccines were well tolerated and elicited expected, short-lived adverse events. Together, these results support further clinical evaluation of the PvDBPII/Matrix-M P. vivax vaccine.
Conflict of interest statement
SJD has consulted to GSK on malaria vaccines, and is an inventor on patent applications relating to adenovirus-based vaccines (PCT/GB2008/001262: Adenoviral Vectors Encoding a Pathogen or Tumour Antigen), and is an inventor on intellectual property licensed by Oxford University Innovation to AstraZeneca. AMM has consulted to GSK on malaria vaccines, and has an immediate family member who is an inventor on patents relating to adenovirus-based vaccines (PCT/GB2008/001262: Adenoviral Vectors Encoding a Pathogen or Tumour Antigen), and is an inventor on intellectual property licensed by Oxford University Innovation to AstraZeneca. CEC is an inventor on patents that relate to binding domains of erythrocyte-binding proteins of
Figures


Update of
-
Impact of a blood-stage vaccine on Plasmodium vivax malaria.medRxiv [Preprint]. 2022 May 30:2022.05.27.22275375. doi: 10.1101/2022.05.27.22275375. medRxiv. 2022. Update in: Sci Transl Med. 2023 Jul 12;15(704):eadf1782. doi: 10.1126/scitranslmed.adf1782. PMID: 35664997 Free PMC article. Updated. Preprint.
References
-
- Organisation WH. World Malaria Report. 2021.
-
- Miller LH, Mason SJ, Clyde DF, Mc MH. Ginniss, The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

