RNF41 orchestrates macrophage-driven fibrosis resolution and hepatic regeneration
- PMID: 37437019
- PMCID: PMC10712730
- DOI: 10.1126/scitranslmed.abq6225
RNF41 orchestrates macrophage-driven fibrosis resolution and hepatic regeneration
Abstract
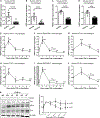
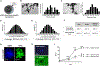
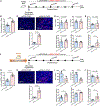
Hepatic inflammation is a common trigger of chronic liver disease. Macrophage activation is a predictive parameter for survival in patients with cirrhosis. Ring finger protein 41 (RNF41) negatively regulates proinflammatory cytokines and receptors; however, the precise involvement of macrophage RNF41 in liver cirrhosis remains unknown. Here, we sought to understand how RNF41 dictates macrophage fate in hepatic fibrosis and repair within the inflammatory milieu. We found that RNF41 expression is down-regulated in CD11b+ macrophages recruited to mouse fibrotic liver and to patient cirrhotic liver regardless of cirrhosis etiology. Prolonged inflammation with TNF-α progressively reduced macrophage RNF41 expression. We designed a macrophage-selective gene therapy with dendrimer-graphite nanoparticles (DGNPs) to explore the influence of macrophage RNF41 restoration and depletion in liver fibrosis and regeneration. RNF41 expression induced in CD11b+ macrophages by DGNP-conjugated plasmids ameliorated liver fibrosis, reduced liver injury, and stimulated hepatic regeneration in fibrotic mice with or without hepatectomy. This therapeutic effect was mainly mediated by the induction of insulin-like growth factor 1. Conversely, depletion of macrophage RNF41 worsened inflammation, fibrosis, hepatic damage, and survival. Our data reveal implications of macrophage RNF41 in the control of hepatic inflammation, fibrosis, and regeneration and provide a rationale for therapeutic strategies in chronic liver disease and potentially other diseases characterized by inflammation and fibrosis.
Figures



Comment in
-
RNF41 reverses liver fibrosis.Nat Rev Drug Discov. 2023 Sep;22(9):697. doi: 10.1038/d41573-023-00125-6. Nat Rev Drug Discov. 2023. PMID: 37528208 No abstract available.
References
-
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS, Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019). - PubMed
-
- Oro D, Yudina T, Fernandez-Varo G, Casals E, Reichenbach V, Casals G, González de la Presa B, Sandalinas S, Carvajal S, Puntes V, Jiménez W, Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 64, 691–698 (2016). - PubMed
-
- Cordoba-Jover B, Arce-Cerezo A, Ribera J, Pauta M, Oró D, Casals G, Fernández-Varo G, Casals E, Puntes V, Jiménez W, Morales-Ruiz M, Cerium oxide nanoparticles improve liver regeneration after acetaminophen-induced liver injury and partial hepatectomy in rats. J. Nanobiotechnol. 17, 112 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

