Discontinuation of Anti-Tumour Necrosis Factor Therapy in Patients with Perianal Fistulizing Crohn's Disease: Individual Participant Data Meta-Analysis of 309 Patients from 12 Studies
- PMID: 37437094
- PMCID: PMC10821706
- DOI: 10.1093/ecco-jcc/jjad118
Discontinuation of Anti-Tumour Necrosis Factor Therapy in Patients with Perianal Fistulizing Crohn's Disease: Individual Participant Data Meta-Analysis of 309 Patients from 12 Studies
Abstract
Background: The risk of relapse after anti-tumour necrosis factor [TNF] therapy discontinuation in Crohn's disease patients with perianal fistulas [pCD] is unclear. We aimed to assess this risk.
Methods: A systematic literature search was conducted to identify cohort studies on the incidence of relapse following anti-TNF discontinuation in pCD patients. Individual participant data were requested from the original study cohorts. Inclusion criteria were age ≥16 years, pCD as a (co)indication for start of anti-TNF therapy, more than three doses, and remission of luminal and pCD at anti-TNF discontinuation. The primary outcome was the cumulative incidence of CD relapse using Kaplan-Meier estimates. Secondary outcomes included response to re-treatment and risk factors associated with relapse as assessed by Cox regression analysis.
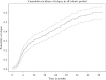
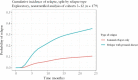
Results: In total, 309 patients from 12 studies in ten countries were included. The median duration of anti-TNF treatment was 14 months [interquartile range 5.8-32.5]. Most patients were treated for pCD without active luminal disease [89%], received first-line anti-TNF therapy [87%], and continued immunomodulatory therapy following anti-TNF discontinuation [78%]. The overall cumulative incidence of relapse was 36% (95% confidence interval [CI] 25-48%) and 42% [95% CI 32-53%] at 1 and 2 years after anti-TNF discontinuation, respectively. Risk factors for relapse included smoking (hazard ratio [HR] 1.5 [1.0, 2.1]) and history of proctitis (HR 1.7 [1.1, 2.5]). The overall re-treatment response rate was 82%.
Conclusions: This individual participant data meta-analysis, on predominantly patients with pCD without active luminal disease and first-line anti-TNF therapy, shows that over half of patients remain in remission 2 years after anti-TNF discontinuation. Therefore, anti-TNF discontinuation may be considered in this subgroup.
Keywords: Crohn’s disease; anti-TNF therapy; discontinuation; perianal fistulizing disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
STBH, DT, ES, RWMP, JM, AL, RB, WL none. CW received grant support from Falk Benelux and Pfizer; received speaker fees from AbbVie, Takeda, Ferring, Dr. Falk Pharma, Hospira, and Pfizer; and served as a consultant for AbbVie, MSD, Takeda, Celgene, Mundipharma, and Janssen. MJ has received education funding from Pfizer, Takeda, Janssen, MSD, Ferring, Abbvie, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, and Norgine. GB has received lecture fees from Abbvie, Ferring, MSD, Takeda, and Pfizer, and consultant fees from Takeda and Janssen. TM has received speaker’s honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer, and Teva. JS has served as national coordinator on studies from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, and Roche/Genentech, and received an unrestricted research grant from Takeda. AA has received consulting fees from Abbvie, Hospira, Takeda, Gilead, and Biocodex as well as lecture fees and travel accommodations from Abbvie, Janssen, Biocodex, Hospira, Ferring, Takeda, and MSD. AA also received advisory board fees from Gilead, Takeda, and AbbvieX. GDH has served as advisor for Abbvie, Ablynx, Alimentiv, Amgen, AM Pharma, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronics, Ferring, DrFALK Pharma, Eli Lilly, Engene, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Immunic, Johnson and Johnson, Lamepro, Lument, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer, Polpharm, Prometheus laboratories/Nestle, Procise diagnostics, Protagonist, Salix, Samsung Bioepis, Sandoz, Setpoint, Shire, Takeda, Tigenix, Tillotts, Topivert, Versant, and Vifor; and received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Takeda, Tillotts, and Vifor. PR has received consultancy fees from Abbvie, Amgen, and Janssen. LG has received consultancies and/or speaker fees from AbbVie, Janssen, MSD, Mundipharma, Takeda, Vifor Pharma, and Zambon. LPB has received grant support, consulting fees, lecture fees, and advisory board fees from AbbVie, Takeda, and MSD, consulting fees, lecture fees, and advisory board fees from Janssen, Ferring, Tillots, Celltrion, Pfizer, and Roche, consulting fees and advisory board fees from Genentech, Pharmacosmos, Sandoz, Celgene, Allergan, Arena, Gilead, and Amgen, consulting fees from Boehringer Ingelheim, Index Pharmaceuticals, and Alma, consulting fees and lecture fees from Biogen and Samsung Bioepis, advisory board fees from Sterna, Nestle, and Enterome, lecture fees from Hikma, and holding stock options in CT-SCOUT. JPG has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine, and Vifor Pharma. AV has participated in advisory board and/or received financial compensation from the following companies: Janssen, Takeda, Abbvie, and Tramedico.
Figures



References
-
- Nielsen OH, Rogler G, Hahnloser D, Thomsen OO.. Diagnosis and management of fistulizing Crohn’s disease. Nat Clin Pract Gastroenterol Hepatol 2009;6:92–106. - PubMed
-
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. . Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. - PubMed
-
- Sands BE, Anderson FH, Bernstein CN, et al. . Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. - PubMed
-
- van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

