Cariprazine in Pediatric Patients with Autism Spectrum Disorder: Results of a Pharmacokinetic, Safety and Tolerability Study
- PMID: 37437109
- PMCID: PMC10458368
- DOI: 10.1089/cap.2022.0097
Cariprazine in Pediatric Patients with Autism Spectrum Disorder: Results of a Pharmacokinetic, Safety and Tolerability Study
Abstract
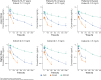
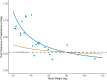
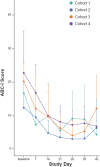
Objective: Cariprazine is a dopamine D3-preferring D3/D2 and serotonin 5-HT1A receptor partial agonist approved to treat adults with schizophrenia and manic/mixed or depressive episodes associated with bipolar I disorder. This study, which is the first to evaluate cariprazine in pediatric patients with autism spectrum disorder (ASD) (including children 5-9 years of age) using an oral solution formulation, evaluated the safety, tolerability, pharmacokinetics (PK), and exploratory efficacy of cariprazine and its two major active metabolites, desmethyl cariprazine (DCAR) and didesmethyl cariprazine (DDCAR). Methods: This clinical pharmacology, open-label, multiple-dose study enrolled 25 pediatric patients from 5 to 17 years of age, who met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for ASD. All patients began treatment with cariprazine 0.5 mg once daily (QD) and underwent a titration over 7 days to maintenance doses of 1.5 or 3 mg QD for patients 13-17 years of age at Screening, 0.75 or 1.5 mg QD for patients 10-12 years of age at Screening, and 0.5 or 1.5 mg QD for patients 5-9 years of age at Screening. After 6 weeks total of dosing, there was a 6-week follow-up period. Study assessments included adverse events (AEs), safety parameters, noncompartmental PK parameters, and exploratory efficacy assessments, including the Aberrant Behavior Checklist-Irritability Subscale (ABC-I), Clinical Global Impressions (CGI-S), Caregiver Global Impressions (CgGI-S), Children's Yale-Brown Obsessive Compulsiveness Scale Modified for ASD (CYBOCS-ASD), Social Responsiveness Scale (SRS), and Vineland Adaptive Behavior Scale (VABS-III). Results: All AEs were mild or moderate in severity. Most frequent treatment-emergent adverse events (TEAEs) were increased weight, increased alanine aminotransferase, increased appetite, dizziness, agitation, and nasal congestion. Increases in weight were not considered clinically meaningful. Two subjects reported extrapyramidal symptom-related TEAEs that resolved without leading to discontinuation. Dose-normalized exposures of all analytes were modestly higher in pediatric patients from 5 to 9 years of age when compared to older patients. Consistent with previous studies, at steady state, the rank of exposure in plasma was DDCAR > cariprazine > DCAR. There was numerical improvement on all exploratory endpoints (ABC-I, CGI-S, CgGI-S, CYBOCS-ASD, SRS, and VABS-III). Conclusions: PK of cariprazine and its metabolites were characterized in pediatric patients with ASD at doses up to 3 mg QD (13-17 years) and 1.5 mg QD (5-12 years). Caripazine treatment was generally well tolerated and results from this study will inform the selection of appropriate pediatric doses for subsequent studies.
Keywords: atypical antipsychotic; autism spectrum disorder; cariprazine; pediatric; pharmacokinetic.
Conflict of interest statement
A.O., T.R., H.V.K., and S.V. are employees of AbbVie and shareholders of AbbVie. K.A.J. and R.R. were investigators for this study and received financial support from AbbVie, which funded the study. P.P.Y. and P.M. are former employees of AbbVie and may be AbbVie shareholders. R.L.F. receives or has received research support, acted as a consultant, and/or has received honoraria from Abbvie, Acadia, Adamas, Afecta, Akili, Alkermes, Allergan, American Academy of Child and Adolescent Psychiatry, American Psychiatric Press, Arbor, Axsome, Idorsia, Intracellular Therapies, Iqvia, Lundbeck, Medavante Prophase, MJH Life Sciences, Neurim, NIH, Novartis, Otsuka, PaxMedica, PCORI, Pfizer, Physicians' Postgraduate Press, Radius, Receptor Life Sciences, Sage, Signant Health, Sunovion, Supernus Pharmaceuticals, Syneos, Takeda, Tris, and Viatris.
Figures

References
-
- APA: Diagnostic and Statistical Manual of Mental Disorders 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
-
- Barnes TR: A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676, 1989. - PubMed
-
- Calabrese JR, Keck PE Jr., Starace A, Lu K, Ruth A, Laszlovszky I, Nemeth G, Durgam S: Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: A double-blind, placebo-controlled study. J Clin Psychiatry 76:284–292, 2015. - PubMed
-
- de Krom M, Staal WG, Ophoff RA, Hendriks J, Buitelaar J, Franke B, de Jonge MV, Bolton P, Collier D, Curran S, van Engeland H, Van Ree JM: A common variant in DRD3 receptor is associated with autism spectrum. disorder. Biol Psychiatry 65:625–630, 2009. - PubMed
-
- Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Nemeth G, Meltzer HY: Cariprazine in acute exacerbation of schizophrenia: A fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry 76:e1574–e1582, 2015b. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
