Integrated Radiology, Pathology, and Pharmacy Program to Accelerate Access to Osimertinib
- PMID: 37437226
- PMCID: PMC10538938
- DOI: 10.1200/OP.23.00031
Integrated Radiology, Pathology, and Pharmacy Program to Accelerate Access to Osimertinib
Abstract
Purpose: Targeted therapy yields superior outcomes relative to genotype-agnostic therapy for patients with epidermal growth factor receptor (EGFR)-mutant lung cancer. Workflows that facilitate timely detection of EGFR mutations and early dispensation of osimertinib can improve management of this disease.
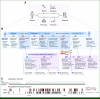
Methods: We developed an Integrated Radiology, Pathology, and Pharmacy Program to minimize delays in initiating osimertinib. The intervention consisted of parallel workflows coupling interventional radiology, surgical pathology, and analysis of nucleic acids from frozen tissue with early pharmacy engagement. We compared time to EGFR testing results and time to treatment for participating patients with those of historical cohorts.
Results: Between January 2020 and December 2021, 222 patients participated in the intervention. The median turnaround time from biopsy to EGFR results was 1 workday. Forty-nine (22%) tumors harbored EGFR exon 19 deletions or EGFR L858R. Thirty-one (63%) patients were prescribed osimertinib via the intervention. The median interval between osimertinib prescription and osimertinib dispensation was 3 days; dispensation occurred within 48 hours for 42% of patients. The median interval between biopsy and osimertinib dispensation was 5 days. Three patients received osimertinib within 24 hours of EGFR results. Compared with patients with EGFR-mutant non-small-cell lung cancer who were diagnosed through routine workflows, the intervention led to a significant reduction in median time between biopsy and EGFR results (1 v 7 days; P < .01) and median time to treatment initiation (5 v 23 days; P < .01).
Conclusion: Combining radiology and pathology workflows with early parallel pharmacy engagement leads to a significant reduction in time to initiating osimertinib. Multidisciplinary integration programs are essential to maximize clinical utility of rapid testing.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


Comment in
-
Expanding the Multidisciplinary Team in Prediagnostic Care of Lung Cancer: How Else Can We Improve Time to Targeted Treatment in Metastatic Non-Small-Cell Lung Cancer?JCO Oncol Pract. 2023 Sep;19(9):694-696. doi: 10.1200/OP.23.00416. Epub 2023 Aug 22. JCO Oncol Pract. 2023. PMID: 37607326 No abstract available.
References
-
- Thai AA, Solomon BJ, Sequist LV, et al. : Lung cancer. Lancet 398:535-554 - PubMed
-
- Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 - PubMed
-
- Peters S, Camidge DR, Shaw AT, et al. : Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829-838, 2017 - PubMed
-
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated. N Engl J Med 382:41-50, 2020 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

