Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase
- PMID: 37437449
- PMCID: PMC10362175
- DOI: 10.1016/j.redox.2023.102807
Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase
Abstract
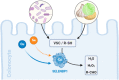
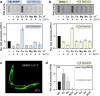
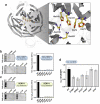
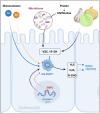
Selenium-binding protein 1 (SELENBP1) was reported to act as a methanethiol oxidase (MTO) in humans, catalyzing the conversion of methanethiol to hydrogen peroxide, hydrogen sulfide and formaldehyde. Here, we identify copper ions as essential to this novel MTO activity. Site-directed mutagenesis of putative copper-binding sites in human SELENBP1 produced as recombinant protein in E. coli resulted in loss of its enzymatic function. On the other hand, the eponymous binding of selenium (as selenite) was no requirement for MTO activity and only moderately increased SELENBP1-catalyzed oxidation of methanethiol. Furthermore, SEMO-1, the SELENBP1 ortholog recently identified in the nematode C. elegans, also requires copper ions, and MTO activity was enhanced or abrogated, respectively, if worms were grown in the presence of cupric chloride or of a Cu chelator. In addition to methanethiol, we identified novel substrates of SELENBP1 from the group of volatile sulfur compounds, ranging from ethanethiol to 1-pentanethiol as well as 2-propene-1-thiol. Gut microbiome-derived methanethiol as well as food-derived volatile sulfur compounds (VSCs) account for malodors that may contribute to extraoral halitosis in humans, if not metabolized properly. As SELENBP1 is particularly abundant in tissues exposed to VSCs, such as colon, liver, and lung, it appears to contribute to copper-dependent VSC degradation.
Keywords: H(2)O(2); H(2)S; MTO; Methanethiol; VSC; Volatile sulfur compounds.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures


References
-
- Steinbrenner H., Speckmann B., Klotz L.O. Selenoproteins: antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016;595:113–119. - PubMed
-
- Bansal M.P., Oborn C.J., Danielson K.G., Medina D. Evidence for two selenium-binding proteins distinct from glutathione peroxidase in mouse liver. Carcinogenesis. 1989;10:541–546. - PubMed
-
- Li T., Yang W., Li M., Byun D.S., Tong C., Nasser S., Zhuang M., Arango D., Mariadason J.M., Augenlicht L.H. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol. Nutr. Food Res. 2008;52:1289–1299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

