Immune sensing of food allergens promotes avoidance behaviour
- PMID: 37437602
- PMCID: PMC10432274
- DOI: 10.1038/s41586-023-06362-4
Immune sensing of food allergens promotes avoidance behaviour
Abstract
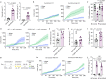
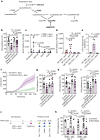
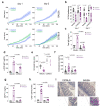
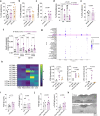
In addition to its canonical function of protection from pathogens, the immune system can also alter behaviour1,2. The scope and mechanisms of behavioural modifications by the immune system are not yet well understood. Here, using mouse models of food allergy, we show that allergic sensitization drives antigen-specific avoidance behaviour. Allergen ingestion activates brain areas involved in the response to aversive stimuli, including the nucleus of tractus solitarius, parabrachial nucleus and central amygdala. Allergen avoidance requires immunoglobulin E (IgE) antibodies and mast cells but precedes the development of gut allergic inflammation. The ability of allergen-specific IgE and mast cells to promote avoidance requires cysteinyl leukotrienes and growth and differentiation factor 15. Finally, a comparison of C57BL/6 and BALB/c mouse strains revealed a strong effect of the genetic background on the avoidance behaviour. These findings thus point to antigen-specific behavioural modifications that probably evolved to promote niche selection to avoid unfavourable environments.
© 2023. The Author(s).
Conflict of interest statement
A.W. received funding from NGM Biopharmaceuticals for research projects unrelated to this study through the Yale Office of Sponsored Projects. The authors declare no other competing interests.
Figures










Update of
-
Immune sensing of food allergens promotes aversive behaviour.bioRxiv [Preprint]. 2023 Jan 20:2023.01.19.524823. doi: 10.1101/2023.01.19.524823. bioRxiv. 2023. Update in: Nature. 2023 Aug;620(7974):643-650. doi: 10.1038/s41586-023-06362-4. PMID: 36712030 Free PMC article. Updated. Preprint.
Comment in
-
The immunology that underlies picky eating.Nature. 2023 Aug;620(7974):497-499. doi: 10.1038/d41586-023-02179-3. Nature. 2023. PMID: 37438630 No abstract available.
-
To eat or not to eat: type 2 immunity controls food avoidance behavior.Trends Immunol. 2023 Sep;44(9):665-667. doi: 10.1016/j.it.2023.07.007. Epub 2023 Aug 15. Trends Immunol. 2023. PMID: 37586904
-
Making good of a tricky start: How IgE and mast cells manage a protective sway in food allergy.Immunity. 2023 Sep 12;56(9):1988-1990. doi: 10.1016/j.immuni.2023.08.010. Immunity. 2023. PMID: 37703829
References
-
- Jackson, K. D., Howie, L. D. & Akinbami, L. J. Trends in Allergic Conditions Among Children: United States, 1997-2011 Data Brief 1–8 (NCHS, 2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

