SARS-CoV-2 post-acute sequelae in previously hospitalised patients: systematic literature review and meta-analysis
- PMID: 37437914
- PMCID: PMC10336551
- DOI: 10.1183/16000617.0254-2022
SARS-CoV-2 post-acute sequelae in previously hospitalised patients: systematic literature review and meta-analysis
Abstract
Background: Many individuals hospitalised with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection experience post-acute sequelae of SARS-CoV-2 infection (PASC), sometimes referred to as "long COVID". Our objective was to conduct a systematic literature review and meta-analysis to identify PASC-associated symptoms in previously hospitalised patients and determine the frequency and temporal nature of PASC.
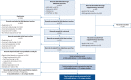
Methods: Searches of MEDLINE, Embase, Cochrane Library (2019-2021), World Health Organization International Clinical Trials Registry Platform and reference lists were performed from November to December 2021. Articles were assessed by two reviewers against eligibility criteria and a risk of bias tool. Symptom data were synthesised by random effects meta-analyses.
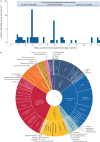
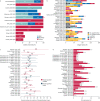
Results: Of 6942 records, 52 studies with at least 100 patients were analysed; ∼70% were Europe-based studies. Most data were from the first wave of the pandemic. PASC symptoms were analysed from 28 days after hospital discharge. At 1-4 months post-acute SARS-CoV-2 infection, the most frequent individual symptoms were fatigue (29.3% (95% CI 20.1-40.6%)) and dyspnoea (19.6% (95% CI 12.8-28.7%)). Many patients experienced at least one symptom at 4-8 months (73.1% (95% CI 44.2-90.3%)) and 8-12 months (75.0% (95% CI 56.4-87.4%)).
Conclusions: A wide spectrum of persistent PASC-associated symptoms were reported over the 1-year follow-up period in a significant proportion of participants. Further research is needed to better define PASC duration and determine whether factors such as disease severity, vaccination and treatments have an impact on PASC.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: J.D. Kelly received consulting fees from Gilead. T. Curteis, A. Rawal, M. Murton, L.J. Clark and Z. Jafry are employees of Costello Medical, which received payment from Gilead Sciences for analytical services for this study. R. Shah-Gupta is an employee of Gilead Sciences. M. Berry is an employee of, and owns stock in, Gilead Sciences. A. Espinueva was an employee of, and owned stock in, Gilead Sciences at the time of the study. L. Chen is an employee of, and owns stock in, Gilead Sciences and owns stocks in Pfizer. M. Abdelghany is an employee of, and owns stock in, Gilead Sciences. D.A. Sweeney has no conflicts of interest to report. J.K. Quint received consulting fees from Gilead, AstraZeneca and Evidera, and received research grants from HDR UK.
Figures



References
-
- Centers for Disease Control and Prevention . Post-COVID conditions: information for healthcare providers. 2021. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-condition... Date last accessed: 9 November 2021.
-
- National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline NG188. 2021. www.nice.org.uk/guidance/ng188 Date last accessed: 9 November 2021.
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard. 2020. https://covid19.who.int Date last accessed: 8 July 2022.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
