Delaying the GABA Shift Indirectly Affects Membrane Properties in the Developing Hippocampus
- PMID: 37438107
- PMCID: PMC10376938
- DOI: 10.1523/JNEUROSCI.0251-23.2023
Delaying the GABA Shift Indirectly Affects Membrane Properties in the Developing Hippocampus
Abstract
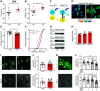
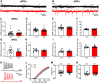
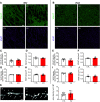
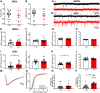
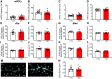
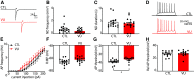
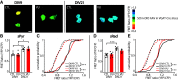
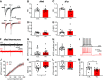
During the first two postnatal weeks, intraneuronal chloride concentrations in rodents gradually decrease, causing a shift from depolarizing to hyperpolarizing GABA responses. The postnatal GABA shift is delayed in rodent models for neurodevelopmental disorders and in human patients, but the impact of a delayed GABA shift on the developing brain remains obscure. Here we examine the direct and indirect consequences of a delayed postnatal GABA shift on network development in organotypic hippocampal cultures made from 6- to 7-d-old mice by treating the cultures for 1 week with VU0463271, a specific inhibitor of the chloride exporter KCC2. We verified that VU treatment delayed the GABA shift and kept GABA signaling depolarizing until DIV9. We found that the structural and functional development of excitatory and inhibitory synapses at DIV9 was not affected after VU treatment. In line with previous studies, we observed that GABA signaling was already inhibitory in control and VU-treated postnatal slices. Surprisingly, 14 d after the VU treatment had ended (DIV21), we observed an increased frequency of spontaneous inhibitory postsynaptic currents in CA1 pyramidal cells, while excitatory currents were not changed. Synapse numbers and release probability were unaffected. We found that dendrite-targeting interneurons in the stratum radiatum had an elevated resting membrane potential, while pyramidal cells were less excitable compared with control slices. Our results show that depolarizing GABA signaling does not promote synapse formation after P7, and suggest that postnatal intracellular chloride levels indirectly affect membrane properties in a cell-specific manner.SIGNIFICANCE STATEMENT During brain development, the action of neurotransmitter GABA shifts from depolarizing to hyperpolarizing. This shift is a thought to play a critical role in synapse formation. A delayed shift is common in rodent models for neurodevelopmental disorders and in human patients, but its consequences for synaptic development remain obscure. Here, we delayed the GABA shift by 1 week in organotypic hippocampal cultures and carefully examined the consequences for circuit development. We find that delaying the shift has no direct effects on synaptic development, but instead leads to indirect, cell type-specific changes in membrane properties. Our data call for careful assessment of alterations in cellular excitability in neurodevelopmental disorders.
Keywords: brain development; chloride; homeostasis; membrane excitability; neurodevelopmental disorders; synapse formation.
Copyright © 2023 the authors.
Figures


Similar articles
-
GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development.J Neurosci. 2006 Nov 8;26(45):11720-5. doi: 10.1523/JNEUROSCI.2887-06.2006. J Neurosci. 2006. PMID: 17093093 Free PMC article.
-
Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus.J Neurophysiol. 1993 May;69(5):1541-55. doi: 10.1152/jn.1993.69.5.1541. J Neurophysiol. 1993. PMID: 8389828
-
Postnatal changes in somatic gamma-aminobutyric acid signalling in the rat hippocampus.Eur J Neurosci. 2008 May;27(10):2515-28. doi: 10.1111/j.1460-9568.2008.06234.x. Eur J Neurosci. 2008. PMID: 18547241
-
The postnatal GABA shift: A developmental perspective.Neurosci Biobehav Rev. 2021 May;124:179-192. doi: 10.1016/j.neubiorev.2021.01.024. Epub 2021 Feb 4. Neurosci Biobehav Rev. 2021. PMID: 33549742 Review.
-
Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus.Crit Rev Neurobiol. 2006;18(1-2):13-23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. Crit Rev Neurobiol. 2006. PMID: 17725505 Review.
Cited by
-
Monitoring Changes in Intracellular Chloride Levels Using the FRET-Based SuperClomeleon Sensor in Organotypic Hippocampal Slices.Bio Protoc. 2025 Mar 5;15(5):e5229. doi: 10.21769/BioProtoc.5229. eCollection 2025 Mar 5. Bio Protoc. 2025. PMID: 40084082 Free PMC article.
-
IL-1β, the first piece to the puzzle of sepsis-related cognitive impairment?Front Neurosci. 2024 Apr 11;18:1370406. doi: 10.3389/fnins.2024.1370406. eCollection 2024. Front Neurosci. 2024. PMID: 38665289 Free PMC article. Review.
References
-
- Awad PN, Amegandjin CA, Szczurkowska J, Carriço JN, Fernandes do Nascimento AS, Baho E, Chattopadhyaya B, Cancedda L, Carmant L, Di Cristo G (2018) KCC2 regulates dendritic spine formation in a brain-region specific and BDNF dependent manner. Cereb Cortex: 28:4049–4062. 10.1093/cercor/bhy198 - DOI - PMC - PubMed
-
- Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M (2016) Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci USA 113:E7287–E7296. 10.1073/pnas.1615330113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
