Decoding a cryptic mechanism of metronidazole resistance among globally disseminated fluoroquinolone-resistant Clostridioides difficile
- PMID: 37438331
- PMCID: PMC10338468
- DOI: 10.1038/s41467-023-39429-x
Decoding a cryptic mechanism of metronidazole resistance among globally disseminated fluoroquinolone-resistant Clostridioides difficile
Abstract
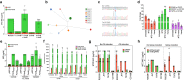
Severe outbreaks and deaths have been linked to the emergence and global spread of fluoroquinolone-resistant Clostridioides difficile over the past two decades. At the same time, metronidazole, a nitro-containing antibiotic, has shown decreasing clinical efficacy in treating C. difficile infection (CDI). Most metronidazole-resistant C. difficile exhibit an unusual resistance phenotype that can only be detected in susceptibility tests using molecularly intact heme. Here, we describe the mechanism underlying this trait. We find that most metronidazole-resistant C. difficile strains carry a T-to-G mutation (which we term PnimBG) in the promoter of gene nimB, resulting in constitutive transcription. Silencing or deleting nimB eliminates metronidazole resistance. NimB is related to Nim proteins that are known to confer resistance to nitroimidazoles. We show that NimB is a heme-dependent flavin enzyme that degrades nitroimidazoles to amines lacking antimicrobial activity. Furthermore, occurrence of the PnimBG mutation is associated with a Thr82Ile substitution in DNA gyrase that confers fluoroquinolone resistance in epidemic strains. Our findings suggest that the pandemic of fluoroquinolone-resistant C. difficile occurring over the past few decades has also been characterized by widespread resistance to metronidazole.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



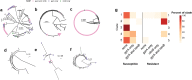
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

