AI-designed NMR spectroscopy RF pulses for fast acquisition at high and ultra-high magnetic fields
- PMID: 37438347
- PMCID: PMC10338431
- DOI: 10.1038/s41467-023-39581-4
AI-designed NMR spectroscopy RF pulses for fast acquisition at high and ultra-high magnetic fields
Abstract
Nuclear magnetic resonance (NMR) spectroscopy is a powerful high-resolution tool for characterizing biomacromolecular structure, dynamics, and interactions. However, the lengthy longitudinal relaxation of the nuclear spins significantly extends the total experimental time, especially at high and ultra-high magnetic field strengths. Although longitudinal relaxation-enhanced techniques have sped up data acquisition, their application has been limited by the chemical shift dispersion. Here we combined an evolutionary algorithm and artificial intelligence to design 1H and 15N radio frequency (RF) pulses with variable phase and amplitude that cover significantly broader bandwidths and allow for rapid data acquisition. We re-engineered the basic transverse relaxation optimized spectroscopy experiment and showed that the RF shapes enhance the spectral sensitivity of well-folded proteins up to 180 kDa molecular weight. These RF shapes can be tailored to re-design triple-resonance experiments for accelerating NMR spectroscopy of biomacromolecules at high fields.
© 2023. The Author(s).
Conflict of interest statement
G.V. and M.V.S. are inventors of the GENETICS-AI software, patent US 11,221,384. G.V. is the founder of Kantika LLC. C.O. declares no competing interests.
Figures
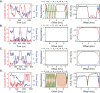




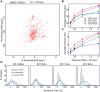
References
-
- Wuthrich, K. NMR of Proteins And Nucleic Acids (Wiley, 1986).
-
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

