Real-world experience of adverse reactions-necessitated rifampicin-sparing treatment for drug-susceptible pulmonary tuberculosis
- PMID: 37438379
- PMCID: PMC10338469
- DOI: 10.1038/s41598-023-38394-1
Real-world experience of adverse reactions-necessitated rifampicin-sparing treatment for drug-susceptible pulmonary tuberculosis
Abstract
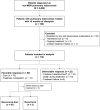
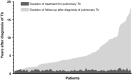
Rifampicin is an important agent for tuberculosis treatment; however, it is often discontinued because of adverse reactions. The treatment regimen then can be administered as that for rifampicin-resistant tuberculosis, which can be toxic. We retrospectively reviewed 114 patients with drug-susceptible pulmonary tuberculosis who discontinued rifampicin due to adverse reactions during an 18 year period at a tertiary referral center, of which 92 (80.7%) exhibited favorable response. Hepatotoxicity was the leading cause of intolerance. Patients with a favorable response were younger and less likely to have comorbidities. The majority of patients were administered four medications during the intensive phase and three to four during the consolidative phase. For those with a favorable response, the median duration of treatment was 10.2 months and the most common intensive regimen was a combination of isoniazid, ethambutol, pyrazinamide, and fluoroquinolone (25%). The most common consolidation regimen was a combination of isoniazid, ethambutol, and fluoroquinolone (22.8%). Among the patients with a favorable response, two (2.2%) experienced recurrence after a follow-up of 3.4 (interquartile range 1.8-6.8) years. For patients with drug-susceptible pulmonary tuberculosis who do not tolerate rifampicin owing to its toxicity, a shorter regimen may be a useful alternative.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
-
Principles of chemotherapy for tuberculosis in national tuberculosis programmes of low- and middle-income countries.Indian J Tuberc. 2020 Dec;67(4S):S16-S22. doi: 10.1016/j.ijtb.2020.11.010. Epub 2020 Nov 28. Indian J Tuberc. 2020. PMID: 33308663 Review.
-
[Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients].Kekkaku. 2001 Jan;76(1):33-43. Kekkaku. 2001. PMID: 11211781 Review. Japanese.
-
Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis.Lancet. 2015 May 2;385(9979):1738-1747. doi: 10.1016/S0140-6736(14)62002-X. Epub 2015 Mar 18. Lancet. 2015. PMID: 25795076 Clinical Trial.
-
Outcomes of pulmonary tuberculosis in patients with discordant phenotypic isoniazid resistance testing.Respir Med. 2017 Dec;133:6-11. doi: 10.1016/j.rmed.2017.11.004. Epub 2017 Nov 8. Respir Med. 2017. PMID: 29173450
Cited by
-
Efficacy of mefloquine and its enantiomers in a murine model of Mycobacterium avium infection.PLoS One. 2024 Sep 30;19(9):e0311167. doi: 10.1371/journal.pone.0311167. eCollection 2024. PLoS One. 2024. PMID: 39348373 Free PMC article.
-
Analysis of nationwide adverse event reports on Isoniazid and Rifampin in tuberculosis prevention and treatment in South Korea.Sci Rep. 2025 Mar 3;15(1):7411. doi: 10.1038/s41598-025-91753-y. Sci Rep. 2025. PMID: 40032948 Free PMC article.
-
Characteristics of and treatment outcomes in rifampicin-intolerant patients.IJTLD Open. 2024 Apr 1;1(4):160-165. doi: 10.5588/ijtldopen.23.0466. eCollection 2024 Apr. IJTLD Open. 2024. PMID: 38988405 Free PMC article.
References
-
- World Health Organization. Global tuberculosis report, <https://www.who.int/publications/i/item/9789240037021> (2021).
-
- Silva SV, Fujiwara PI, Frieden TR. Rates and risk factors for discontinuation of rifampicin. Int. J. Tuberc. Lung Dis. 2000;4:118–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

