Abrupt perturbation and delayed recovery of the vaginal ecosystem following childbirth
- PMID: 37438386
- PMCID: PMC10338445
- DOI: 10.1038/s41467-023-39849-9
Abrupt perturbation and delayed recovery of the vaginal ecosystem following childbirth
Erratum in
-
Publisher Correction: Abrupt perturbation and delayed recovery of the vaginal ecosystem following childbirth.Nat Commun. 2024 Feb 26;15(1):1744. doi: 10.1038/s41467-024-46160-8. Nat Commun. 2024. PMID: 38409135 Free PMC article. No abstract available.
Abstract
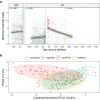
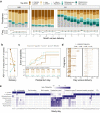
The vaginal ecosystem is closely tied to human health and reproductive outcomes, yet its dynamics in the wake of childbirth remain poorly characterized. Here, we profile the vaginal microbiota and cytokine milieu of participants sampled longitudinally throughout pregnancy and for at least one year postpartum. We show that delivery, regardless of mode, is associated with a vaginal pro-inflammatory cytokine response and the loss of Lactobacillus dominance. By contrast, neither the progression of gestation nor the approach of labor strongly altered the vaginal ecosystem. At 9.5-months postpartum-the latest timepoint at which cytokines were assessed-elevated inflammation coincided with vaginal bacterial communities that had remained perturbed (highly diverse) from the time of delivery. Time-to-event analysis indicated a one-year postpartum probability of transitioning to Lactobacillus dominance of 49.4%. As diversity and inflammation declined during the postpartum period, dominance by L. crispatus, the quintessential health-associated commensal, failed to return: its prevalence before, immediately after, and one year after delivery was 41%, 4%, and 9%, respectively. Revisiting our pre-delivery data, we found that a prior live birth was associated with a lower odds of L. crispatus dominance in pregnant participants-an outcome modestly tempered by a longer ( > 18-month) interpregnancy interval. Our results suggest that reproductive history and childbirth in particular remodel the vaginal ecosystem and that the timing and degree of recovery from delivery may help determine the subsequent health of the woman and of future pregnancies.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mesiano, S. Chapter 11. Endocrinology of Human Pregnancy and Fetal-Placental Neuroendocrine Development. in Yen & Jaffe’s Reproductive Endocrinology, 8th Edition (eds. J. F. Strauss & R. L. Barbieri) 256–284 (Elsevier, 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

