Co-transplantation of autologous Treg cells in a cell therapy for Parkinson's disease
- PMID: 37438521
- PMCID: PMC12012854
- DOI: 10.1038/s41586-023-06300-4
Co-transplantation of autologous Treg cells in a cell therapy for Parkinson's disease
Abstract
The specific loss of midbrain dopamine neurons (mDANs) causes major motor dysfunction in Parkinson's disease, which makes cell replacement a promising therapeutic approach1-4. However, poor survival of grafted mDANs remains an obstacle to successful clinical outcomes5-8. Here we show that the surgical procedure itself (referred to here as 'needle trauma') triggers a profound host response that is characterized by acute neuroinflammation, robust infiltration of peripheral immune cells and brain cell death. When midbrain dopamine (mDA) cells derived from human induced pluripotent stem (iPS) cells were transplanted into the rodent striatum, less than 10% of implanted tyrosine hydroxylase (TH)+ mDANs survived at two weeks after transplantation. By contrast, TH- grafted cells mostly survived. Notably, transplantation of autologous regulatory T (Treg) cells greatly modified the response to needle trauma, suppressing acute neuroinflammation and immune cell infiltration. Furthermore, intra-striatal co-transplantation of Treg cells and human-iPS-cell-derived mDA cells significantly protected grafted mDANs from needle-trauma-associated death and improved therapeutic outcomes in rodent models of Parkinson's disease with 6-hydroxydopamine lesions. Co-transplantation with Treg cells also suppressed the undesirable proliferation of TH- grafted cells, resulting in more compact grafts with a higher proportion and higher absolute numbers of TH+ neurons. Together, these data emphasize the importance of the initial inflammatory response to surgical injury in the differential survival of cellular components of the graft, and suggest that co-transplanting autologous Treg cells effectively reduces the needle-trauma-induced death of mDANs, providing a potential strategy to achieve better clinical outcomes for cell therapy in Parkinson's disease.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures


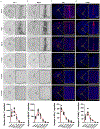


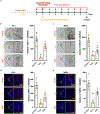


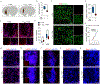
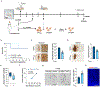





Comment in
-
Regulatory T cells aid stem-cell therapy for Parkinson's disease.Nature. 2023 Jul;619(7970):470-472. doi: 10.1038/d41586-023-02177-5. Nature. 2023. PMID: 37438628 No abstract available.
References
-
- Parmar M, Grealish S & Henchcliffe C The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci 21, 103–115 (2020). - PubMed
-
- Barker RA, Drouin-Ouellet J & Parmar M Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol 11, 492–503 (2015). - PubMed
-
- Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med 279, 30–40 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

