A contemporary assessment of total neoadjuvant therapy (TNT) protocols for locally advanced rectal cancer: adoption and expert perspectives at German Cancer Society (DKG)-certified colorectal cancer centers
- PMID: 37438538
- PMCID: PMC10465655
- DOI: 10.1007/s00432-023-05139-6
A contemporary assessment of total neoadjuvant therapy (TNT) protocols for locally advanced rectal cancer: adoption and expert perspectives at German Cancer Society (DKG)-certified colorectal cancer centers
Abstract
Purpose: The treatment paradigm for locally advanced rectal cancer (LARC) is shifting toward the total neoadjuvant therapy (TNT) concept, which administered systemic chemotherapy in the neoadjuvant setting, either before or after chemoradiotherapy (CRT) or short-course radiotherapy (SCRT). First results have shown higher pathologic complete response (pCR) rates and a favorable impact on disease-free survival (DFS). Our study aimed to evaluate the current clinical practice and expert opinion regarding TNT for locally advanced rectal cancer across DKG (German Cancer Society)-certified colorectal cancer centers.
Methods: A comprehensive online questionnaire, constituted of 14 TNT-focused queries targeting patients with locally advanced rectal cancer, was conducted among DKG-certified colorectal cancer centers registered within the database of the Addz (Arbeitsgemeinschaft Deutscher Darmzentren) between December 2022 and January 2023.
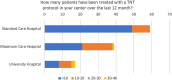
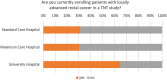
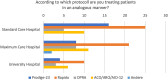
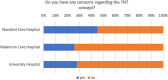
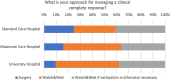
Results: A significant majority (68%) indicated that they treated between 0 and 10 patients using a TNT protocol. Only a third (36%) of these centers participated in patient enrollment for a TNT study. Despite this, 84% of centers reported treating patients in a manner analogous to a TNT study, with the RAPIDO regimen being the most prevalent approach, employed by 60% of the respondents. The decision to adopt a TNT approach was primarily influenced by factors, such as the lower third of the rectum (93% of centers), cT4 stage (86% of centers), and a positive circumferential resection margin (80% of centers). Regarding concerns, 65% of the survey respondents expressed no reservations about the TNT concept, while 35% had concerns. In particular, there appears to be disagreement and uncertainty in regard to a clinical complete response and the "Watch and Wait" approach. While some centers adopt the watch-and-wait approach (42%), others only utilize it when extirpation is otherwise necessary (39%), and a portion still proceeds with surgery as initially planned (19%). The survey also addressed unmet needs, which were elaborated in the free-text responses. Overall, there was high interest in participating in planned observational studies.
Conclusions: This study presents an overview of current clinical practice and unmet needs within DKG-certified German colorectal cancer centers. It is noteworthy that total neoadjuvant therapy (TNT) is predominantly performed outside of clinical trials. Moreover, across the centers, there is significant heterogeneity in handling clinical complete response and adopting the "watch and wait" approach. Further research is needed to establish standardization in the care of locally advanced rectal cancer.
Keywords: DKG-certified colorectal cancer centers; Locally advanced rectal cancer; Rectal cancer; TNT; Total neoadjuvant therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


Similar articles
-
Survival After Induction Chemotherapy and Chemoradiation Versus Chemoradiation and Adjuvant Chemotherapy for Locally Advanced Rectal Cancer.Oncologist. 2022 May 6;27(5):380-388. doi: 10.1093/oncolo/oyac025. Oncologist. 2022. PMID: 35278070 Free PMC article.
-
[Analysis on efficacy and safety of total neoadjuvant therapy in patients with locally advanced rectal cancer with high risk factors].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Apr 25;22(4):349-356. doi: 10.3760/cma.j.issn.1671-0274.2019.04.007. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31054549 Chinese.
-
[Rectum-preserving surgery after consolidation neoadjuvant therapy or totally neoadjuvant therapy for low rectal cancer: a preliminary report].Zhonghua Wei Chang Wai Ke Za Zhi. 2020 Mar 25;23(3):281-288. doi: 10.3760/cma.j.cn.441530-20200228-00096. Zhonghua Wei Chang Wai Ke Za Zhi. 2020. PMID: 32192308 Chinese.
-
Implications of recent neoadjuvant clinical trials on the future practice of radiotherapy in locally advanced rectal cancer.World J Gastroenterol. 2023 Feb 14;29(6):1011-1025. doi: 10.3748/wjg.v29.i6.1011. World J Gastroenterol. 2023. PMID: 36844136 Free PMC article. Review.
-
Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Dec 1;3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097. JAMA Netw Open. 2020. PMID: 33326026 Free PMC article.
Cited by
-
Novel regimens and treatment strategies in neoadjuvant therapy for colorectal cancer: A systematic review.Int J Health Sci (Qassim). 2024 Sep-Oct;18(5):43-58. Int J Health Sci (Qassim). 2024. PMID: 39282125 Free PMC article. Review.
-
Assessing the practice of total neoadjuvant therapy for rectal cancer: an online survey among radiation oncology departments in Germany and German-speaking regions of Austria and Switzerland.Clin Exp Med. 2024 Oct 19;24(1):242. doi: 10.1007/s10238-024-01495-w. Clin Exp Med. 2024. PMID: 39427077 Free PMC article.
References
-
- Bahadoer RR et al (2021) Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 22(1):29–42 - PubMed
-
- Breugom AJ et al (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16(2):200–207 - PubMed
-
- Conroy T et al (2021) Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22(5):702–715 - PubMed
-
- Donnelly M et al (2023) Total neoadjuvant therapy versus standard neoadjuvant treatment strategies for the management of locally advanced rectal cancer: network meta-analysis of randomized clinical trials. Br J Surg. 10.1093/bjs/znad177 - PubMed
-
- Fokas E et al (2019) Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol 37(34):3212–3222 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

