Hypothermic oxygenated perfusion attenuates DCD liver ischemia-reperfusion injury by activating the JAK2/STAT3/HAX1 pathway to regulate endoplasmic reticulum stress
- PMID: 37438690
- PMCID: PMC10337067
- DOI: 10.1186/s11658-023-00466-5
Hypothermic oxygenated perfusion attenuates DCD liver ischemia-reperfusion injury by activating the JAK2/STAT3/HAX1 pathway to regulate endoplasmic reticulum stress
Abstract
Background: Hepatic ischemia-reperfusion injury (IRI) in donation after cardiac death (DCD) donors is a major determinant of transplantation success. Endoplasmic reticulum (ER) stress plays a key role in hepatic IRI, with potential involvement of the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathway and the antiapoptotic protein hematopoietic-lineage substrate-1-associated protein X-1 (HAX1). In this study, we aimed to investigate the effects of hypothermic oxygenated perfusion (HOPE), an organ preservation modality, on ER stress and apoptosis during hepatic IRI in a DCD rat model.
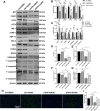
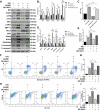
Methods: To investigate whether HOPE could improve IRI in DCD livers, levels of different related proteins were examined by western blotting and quantitative real-time polymerase chain reaction. Further expression analyses, immunohistochemical analyses, immunofluorescence staining, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining, and transmission electron microscopy were conducted to analyze the effects of HOPE on ER stress and apoptosis. To clarify the role of the JAK2/STAT3 pathway and HAX1 in this process, AG490 inhibitor, JAX1 plasmid transfection, co-immunoprecipitation (CO-IP), and flow cytometry analyses were conducted.
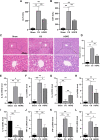
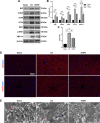
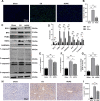
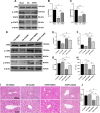
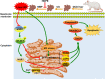
Results: HOPE reduced liver injury and inflammation while alleviating ER stress and apoptosis in the DCD rat model. Mechanistically, HOPE inhibited unfolded protein responses by activating the JAK2/STAT3 pathway, thus reducing ER stress and apoptosis. Moreover, the activated JAK2/STAT3 pathway upregulated HAX1, promoting the interaction between HAX1 and SERCA2b to maintain ER calcium homeostasis. Upregulated HAX1 also modulated ER stress and apoptosis by inhibiting the inositol-requiring enzyme 1 (IRE1) pathway.
Conclusions: JAK2/STAT3-mediated upregulation of HAX1 during HOPE alleviates hepatic ER stress and apoptosis, indicating the JAK2/STAT3/HAX1 pathway as a potential target for IRI management during DCD liver transplantation.
Keywords: DCD; Endoplasmic reticulum stress; HAX1; HOPE; IRI; JAK2/STAT3; Liver transplantation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Hypothermic oxygenated perfusion combined with TJ-M2010-5 alleviates hepatic ischemia-reperfusion injury in donation after circulatory death.Int Immunopharmacol. 2022 Apr;105:108541. doi: 10.1016/j.intimp.2022.108541. Epub 2022 Jan 18. Int Immunopharmacol. 2022. PMID: 35063749
-
Hypothermic oxygenated perfusion inhibits HECTD3-mediated TRAF3 polyubiquitination to alleviate DCD liver ischemia-reperfusion injury.Cell Death Dis. 2021 Feb 24;12(2):211. doi: 10.1038/s41419-021-03493-2. Cell Death Dis. 2021. PMID: 33627626 Free PMC article.
-
Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress.Acta Pharmacol Sin. 2016 Mar;37(3):354-67. doi: 10.1038/aps.2015.136. Epub 2016 Jan 25. Acta Pharmacol Sin. 2016. PMID: 26806299 Free PMC article.
-
The research development of STAT3 in hepatic ischemia-reperfusion injury.Front Immunol. 2023 Jan 24;14:1066222. doi: 10.3389/fimmu.2023.1066222. eCollection 2023. Front Immunol. 2023. PMID: 36761734 Free PMC article. Review.
-
HAX1: A versatile, intrinsically disordered regulatory protein.Biochim Biophys Acta Mol Cell Res. 2023 Oct;1870(7):119538. doi: 10.1016/j.bbamcr.2023.119538. Epub 2023 Jul 15. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 37454914 Review.
Cited by
-
HOPE and AMPK activation reduce reperfusion injury and metabolic dysfunction in primate steatotic liver grafts.Sci Rep. 2025 Apr 6;15(1):11762. doi: 10.1038/s41598-025-96265-3. Sci Rep. 2025. PMID: 40189683 Free PMC article.
-
VCA supercooling in a swine partial hindlimb model.Sci Rep. 2024 Jun 1;14(1):12618. doi: 10.1038/s41598-024-63041-8. Sci Rep. 2024. PMID: 38824189 Free PMC article.
-
Real-time monitoring of mitochondrial oxygenation during machine perfusion using resonance Raman spectroscopy predicts organ function.Sci Rep. 2024 Mar 27;14(1):7328. doi: 10.1038/s41598-024-57773-w. Sci Rep. 2024. PMID: 38538723 Free PMC article.
-
Feedback Loops Shape Oxidative and Immune Interactions in Hepatic Ischemia-Reperfusion Injury.Antioxidants (Basel). 2025 Jul 31;14(8):944. doi: 10.3390/antiox14080944. Antioxidants (Basel). 2025. PMID: 40867840 Free PMC article. Review.
-
Hypothermic oxygenated perfusion inhibits CLIP1-mediated TIRAP ubiquitination via TFPI2 to reduce ischemia‒reperfusion injury of the fatty liver.Exp Mol Med. 2024 Dec;56(12):2588-2601. doi: 10.1038/s12276-024-01350-8. Epub 2024 Dec 2. Exp Mol Med. 2024. PMID: 39617791 Free PMC article.
References
-
- Sousa Da Silva RX, Weber A, Dutkowski P, Clavien PA. Machine perfusion in liver transplantation. Hepatology. 2022;76(5):1531–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

