Booster vaccinations and Omicron: the effects on SARS-CoV-2 antibodies in Dutch blood donors
- PMID: 37438703
- PMCID: PMC10339593
- DOI: 10.1186/s12879-023-08448-w
Booster vaccinations and Omicron: the effects on SARS-CoV-2 antibodies in Dutch blood donors
Abstract
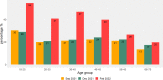
Introduction: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) booster vaccination campaign and the emergence of SARS-CoV-2 Omicron variants impact the prevalence and levels of SARS-CoV-2 antibodies in the Netherlands. In this study we determined antibody levels across age groups, the impact of Omicron variant infections, and the effect of booster vaccinations on antibody levels.
Methods: In September and December 2021 and in February 2022, over 2000 Dutch blood donors were tested for presence of SARS-CoV-2 antibodies. Donations were selected based on age, sex, and region of residence, to provide an optimal coverage and representation of the Dutch population.
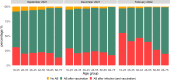
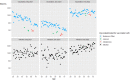
Results: Levels of vaccination-induced spike antibodies decreased over time in all age groups. Donors vaccinated with Janssen or AstraZeneca had significantly lower antibody levels than donors vaccinated with Pfizer or Moderna vaccine. Boostering with an mRNA vaccine elevated antibody levels in all age-groups irrespective of the initial vaccine. In donors aged < 56 years, the proportion of infected donors almost doubled between December 2021 and February 2022.
Conclusion: The booster vaccination campaign increased antibody levels in all age-groups. After a booster vaccination, donors initially vaccinated with AstraZeneca or Janssen vaccine showed antibody levels similar to donors initially vaccinated with an mRNA vaccine. The emergence of the SARS-CoV-2 Omicron variant in the Netherlands caused a substantial increase in donors with infection-induced antibodies, especially among younger donors.
Keywords: Antibodies; Antibody waning; Blood donor; Infection; SARS-CoV-2; Sero-surveillance; Vaccination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
[COVID-19 infections and effectiveness of the vaccination among healthcare workers].Orv Hetil. 2023 Feb 5;164(5):163-171. doi: 10.1556/650.2023.32709. Print 2023 Feb 5. Orv Hetil. 2023. PMID: 36739547 Hungarian.
-
Efficacy of the neutralizing antibodies after the booster dose on SARS-CoV-2 Omicron variant and a two-year longitudinal antibody study on Wild Type convalescents.Int Immunopharmacol. 2023 Jun;119:110151. doi: 10.1016/j.intimp.2023.110151. Epub 2023 Apr 5. Int Immunopharmacol. 2023. PMID: 37044040 Free PMC article.
-
Disease severity and efficacy of homologous vaccination among patients infected with SARS-CoV-2 Delta or Omicron VOCs, compared to unvaccinated using main biomarkers.J Med Virol. 2022 Dec;94(12):5867-5876. doi: 10.1002/jmv.28098. Epub 2022 Sep 9. J Med Virol. 2022. PMID: 36029103 Free PMC article.
-
Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland-A multicentre cohort study.PLoS Med. 2022 Nov 7;19(11):e1004125. doi: 10.1371/journal.pmed.1004125. eCollection 2022 Nov. PLoS Med. 2022. PMID: 36342956 Free PMC article.
-
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis.Front Public Health. 2022 Jul 13;10:940956. doi: 10.3389/fpubh.2022.940956. eCollection 2022. Front Public Health. 2022. PMID: 35910897 Free PMC article.
Cited by
-
SARS-CoV-2 infection and vaccination status in six ethnic groups in Amsterdam, The Netherlands, May to November 2022.Epidemiol Infect. 2025 Jan 23;153:e23. doi: 10.1017/S0950268825000056. Epidemiol Infect. 2025. PMID: 39844374 Free PMC article.
-
SARS-CoV-2 vaccination in Canadian blood donors: Insight into donor representativeness of the general population.Vaccine X. 2024 May 12;18:100498. doi: 10.1016/j.jvacx.2024.100498. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38800670 Free PMC article.
References
-
- RIVM. Epidemiologische situatie van SARS-CoV-2 in Nederland [Internet]. Rijksinstituut voor Volksgezondheid en Milieu. ; 2021. Available from: https://www.rivm.nl/coronavirus-covid-19/actueel/wekelijkse-update-epide....
-
- Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MPHS et al. Clinical and Virological Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin Infect Dis [Internet]. 2022 Jul 1;75(1):e1128–36. Available from: 10.1093/cid/ciab721. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

