Earth-friendly micellar UPLC technique for determination of four hypoglycemic drugs in different pharmaceutical dosage forms and spiked human plasma
- PMID: 37438757
- PMCID: PMC10339510
- DOI: 10.1186/s13065-023-00983-6
Earth-friendly micellar UPLC technique for determination of four hypoglycemic drugs in different pharmaceutical dosage forms and spiked human plasma
Abstract
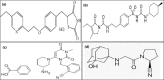
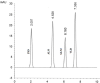
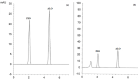
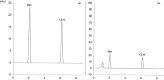
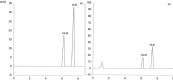
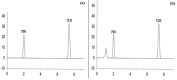
A novel, sensitive, and green micellar UPLC method was proposed and validated for the simultaneous determination of four hypoglycemic agents used in type II diabetes mellitus treatment namely, pioglitazone, alogliptin, glimepiride, and vildagliptin. The developed UPLC method was successfully applied for quantitative analysis of these drugs in bulk, in pharmaceutical formulations, and in spiked human plasma. Chromatographic separation was carried out on a Kinetex® 1.7 μm XB-C18 100 Å (50 × 2.1 mm) column, using a degassed and filtered mixture of (0.1 M SDS- 0.3% triethyl amine- 0.1% phosphoric acid (pH 6)) and n-propanol (85:15 v/v), at a flow rate of 0.2 mL/min. The experimental conditions of the suggested method were well investigated and optimized. The newly developed micellar UPLC method is capable of determining different dosage forms at the same time with the same solvents, saving time and effort. The method was found to be efficiently applicable in spiked human plasma and could be extended to study the pharmacokinetics of the cited drugs in real human plasma samples. The greenness of the developed method was evaluated by applying the Eco-scale scoring tool, which verified the excellent greenness of the analytical method.
Keywords: Alogliptin; Glimepiride; Micellar UPLC; Pioglitazone; Spiked human plasma; Vildagliptin..
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Eco-friendly micellar HPLC approach for simultaneous estimation of combination therapy for hidradenitis suppurativa: Applications to spiked human plasma and different dosage forms.Arch Pharm (Weinheim). 2024 Feb;357(2):e2300509. doi: 10.1002/ardp.202300509. Epub 2023 Nov 8. Arch Pharm (Weinheim). 2024. PMID: 37939289
-
Assessment of the greenness of new stability indicating micellar UPLC and HPTLC methods for determination of tenofovir alafenamide in dosage forms.J Chromatogr Sci. 2021 Oct 29;59(10):909-922. doi: 10.1093/chromsci/bmaa143. J Chromatogr Sci. 2021. PMID: 33529317
-
A Green Approach: Optimization of the UPLC Method Using DoE Software for Concurrent Quantification of Pioglitazone and Dapagliflozin in a SNEDDS Formulation for the Treatment of Diabetes.ACS Omega. 2024 Nov 1;9(45):45011-45024. doi: 10.1021/acsomega.4c04927. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554438 Free PMC article.
-
Development and validation of a repharsed phase- HPLC method for simultaneous determination of rosiglitazone and glimepiride in combined dosage forms and human plasma.Chem Cent J. 2012 Jan 26;6(1):9. doi: 10.1186/1752-153X-6-9. Chem Cent J. 2012. PMID: 22277722 Free PMC article.
-
Eco-friendly estimation of isosorbide dinitrate and hydralazine hydrochloride using Green Analytical Quality by Design-based UPLC Method.RSC Adv. 2021 Sep 7;11(45):27820-27831. doi: 10.1039/d1ra04843k. eCollection 2021 Aug 16. RSC Adv. 2021. PMID: 35480770 Free PMC article.
References
-
- Bastaki S. Diabetes mellitus and its treatment. Dubai Diabetes and Endocrinology Journal. 2005;13:111–34.
-
- The United States pharmacopeia . National formulary. Rockville (MD): United States Pharmacopeial Convention; 2021.
-
- Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001;121:13–8. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
