Adenosine metabolized from extracellular ATP ameliorates organ injury by triggering A2BR signaling
- PMID: 37438813
- PMCID: PMC10339538
- DOI: 10.1186/s12931-023-02486-3
Adenosine metabolized from extracellular ATP ameliorates organ injury by triggering A2BR signaling
Abstract
Background: Trauma and a subsequent hemorrhagic shock (T/HS) result in insufficient oxygen delivery to tissues and multiple organ failure. Extracellular adenosine, which is a product of the extracellular degradation of adenosine 5' triphosphate (ATP) by the membrane-embedded enzymes CD39 and CD73, is organ protective, as it participates in signaling pathways, which promote cell survival and suppress inflammation through adenosine receptors including the A2BR. The aim of this study was to evaluate the role of CD39 and CD73 delivering adenosine to A2BRs in regulating the host's response to T/HS.
Methods: T/HS shock was induced by blood withdrawal from the femoral artery in wild-type, global knockout (CD39, CD73, A2BR) and conditional knockout (intestinal epithelial cell-specific deficient VillinCre-A2BRfl/fl) mice. At 3 three hours after resuscitation, blood and tissue samples were collected to analyze organ injury.
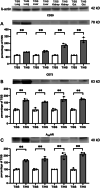
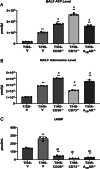
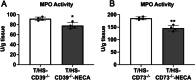
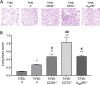
Results: T/HS upregulated the expression of CD39, CD73, and the A2BR in organs. ATP and adenosine levels increased after T/HS in bronchoalveolar lavage fluid. CD39, CD73, and A2BR mimics/agonists alleviated lung and liver injury. Antagonists or the CD39, CD73, and A2BR knockout (KO) exacerbated lung injury, inflammatory cytokines, and chemokines as well as macrophage and neutrophil infiltration and accumulation in the lung. Agonists reduced the levels of the liver enzymes aspartate transferase and alanine transaminase in the blood, whereas antagonist administration or CD39, CD73, and A2BR KO enhanced enzyme levels. In addition, intestinal epithelial cell-specific deficient VillinCre-A2BRfl/fl mice showed increased intestinal injury compared to their wild-type VillinCre controls.
Conclusion: In conclusion, the CD39-CD73-A2BR axis protects against T/HS-induced multiple organ failure.
Keywords: A2BR; Acute lung injury; Adenosine; CD39; CD73; Trauma hemorrhagic shock.
© 2023. The Author(s).
Conflict of interest statement
G.H. owns stock in Purine Pharmaceuticals, Inc. S.C.R. is the scientific cofounder of Purinomia and a consultant to SynLogic and eGenesis. The other authors have no financial conflict of interest.
Figures



References
-
- Cannon JW, Hemorrhagic Shock N Engl J Med. 2018;19:1852–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

