Host obesity alters the ovarian tumor immune microenvironment and impacts response to standard of care chemotherapy
- PMID: 37438818
- PMCID: PMC10337170
- DOI: 10.1186/s13046-023-02740-y
Host obesity alters the ovarian tumor immune microenvironment and impacts response to standard of care chemotherapy
Abstract
Background: The majority of women with epithelial ovarian cancer (OvCa) are diagnosed with metastatic disease, resulting in a poor 5-year survival of 31%. Obesity is a recognized non-infectious pandemic that increases OvCa incidence, enhances metastatic success and reduces survival. We have previously demonstrated a link between obesity and OvCa metastatic success in a diet-induced obesity mouse model wherein a significantly enhanced tumor burden was associated with a decreased M1/M2 tumor-associated macrophage ratio (Liu Y et al. Can, Res. 2015; 75:5046-57).
Methods: The objective of this study was to use pre-clinical murine models of diet-induced obesity to evaluate the effect of a high fat diet (HFD) on response to standard of care chemotherapy and to assess obesity-associated changes in the tumor microenvironment. Archived tumor tissues from ovarian cancer patients of defined body mass index (BMI) were also evaluated using multiplexed immunofluorescence analysis of immune markers.
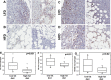
Results: We observed a significantly diminished response to standard of care paclitaxel/carboplatin chemotherapy in HFD mice relative to low fat diet (LFD) controls. A corresponding decrease in the M1/M2 macrophage ratio and enhanced tumor fibrosis were observed both in murine DIO studies and in human tumors from women with BMI > 30.
Conclusions: Our data suggest that the reported negative impact of obesity on OvCa patient survival may be due in part to the effect of the altered M1/M2 tumor-associated macrophage ratio and enhanced fibrosis on chemosensitivity. These data demonstrate a contribution of host obesity to ovarian tumor progression and therapeutic response and support future combination strategies targeting macrophage polarization and/or fibrosis in the obese host.
Keywords: Fibrosis; High fat diet; Obesity; Ovarian cancer; Tumor-associated macrophage.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

