Lipofuscin Granule Accumulation Requires Autophagy Activation
- PMID: 37438887
- PMCID: PMC10440269
- DOI: 10.14348/molcells.2023.0019
Lipofuscin Granule Accumulation Requires Autophagy Activation
Abstract
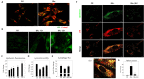
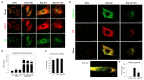
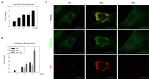
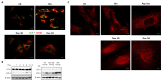
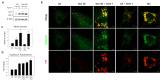
Lipofuscins are oxidized lipid and protein complexes that accumulate during cellular senescence and tissue aging, regarded as markers for cellular oxidative damage, tissue aging, and certain aging-associated diseases. Therefore, understanding their cellular biological properties is crucial for effective treatment development. Through traditional microscopy, lipofuscins are readily observed as fluorescent granules thought to accumulate in lysosomes. However, lipofuscin granule formation and accumulation in senescent cells are poorly understood. Thus, this study examined lipofuscin accumulation in human fibroblasts exposed to various stressors. Our results substantiate that in glucose-starved or replicative senescence cells, where elevated oxidative stress levels activate autophagy, lipofuscins predominately appear as granules that co-localize with autolysosomes due to lysosomal acidity or impairment. Meanwhile, autophagosome formation is attenuated in cells experiencing oxidative stress induced by a doxorubicin pulse and chase, and lipofuscin fluorescence granules seldom manifest in the cytoplasm. As Torin-1 treatment activates autophagy, granular lipofuscins intensify and dominate, indicating that autophagy activation triggers their accumulation. Our results suggest that high oxidative stress activates autophagy but fails in lipofuscin removal, leaving an abundance of lipofuscin-filled impaired autolysosomes, referred to as residual bodies. Therefore, future endeavors in treating lipofuscin pathology-associated diseases and dysfunctions through autophagy activation demand meticulous consideration.
Keywords: autolysosome; autophagy; cellular senescence; lipofuscin; lipofuscin granule.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Boulton M., Davies S., Ellis S. Lipofuscin turnover. Invest. Ophthalmol. Vis. Sci. 1999;40:1887–1888. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

