Variability in Cochlear Implantation Outcomes in a Large German Cohort With a Genetic Etiology of Hearing Loss
- PMID: 37438890
- PMCID: PMC10583923
- DOI: 10.1097/AUD.0000000000001386
Variability in Cochlear Implantation Outcomes in a Large German Cohort With a Genetic Etiology of Hearing Loss
Abstract
Objectives: The variability in outcomes of cochlear implantation is largely unexplained, and clinical factors are not sufficient for predicting performance. Genetic factors have been suggested to impact outcomes, but the clinical and genetic heterogeneity of hereditary hearing loss makes it difficult to determine and interpret postoperative performance. It is hypothesized that genetic mutations that affect the neuronal components of the cochlea and auditory pathway, targeted by the cochlear implant (CI), may lead to poor performance. A large cohort of CI recipients was studied to verify this hypothesis.
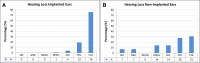
Design: This study included a large German cohort of CI recipients (n = 123 implanted ears; n = 76 probands) with a definitive genetic etiology of hearing loss according to the American College of Medical Genetics (ACMG)/Association for Molecular Pathology (AMP) guidelines and documented postoperative audiological outcomes. All patients underwent preoperative clinical and audiological examinations. Postoperative CI outcome measures were based on at least 1 year of postoperative audiological follow-up for patients with postlingual hearing loss onset (>6 years) and 5 years for children with congenital or pre/perilingual hearing loss onset (≤6 years). Genetic analysis was performed based on three different methods that included single-gene screening, custom-designed hearing loss gene panel sequencing, targeting known syndromic and nonsyndromic hearing loss genes, and whole-genome sequencing.
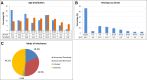
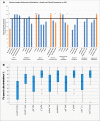
Results: The genetic diagnosis of the 76 probands in the genetic cohort involved 35 genes and 61 different clinically relevant (pathogenic, likely pathogenic) variants. With regard to implanted ears (n = 123), the six most frequently affected genes affecting nearly one-half of implanted ears were GJB2 (21%; n = 26), TMPRSS3 (7%; n = 9), MYO15A (7%; n = 8), SLC26A4 (5%; n = 6), and LOXHD1 and USH2A (each 4%; n = 5). CI recipients with pathogenic variants that influence the sensory nonneural structures performed at or above the median level of speech performance of all ears at 70% [monosyllable word recognition score in quiet at 65 decibels sound pressure level (SPL)]. When gene expression categories were compared to demographic and clinical categories (total number of compared categories: n = 30), mutations in genes expressed in the spiral ganglion emerged as a significant factor more negatively affecting cochlear implantation outcomes than all clinical parameters. An ANOVA of a reduced set of genetic and clinical categories (n = 10) identified five detrimental factors leading to poorer performance with highly significant effects ( p < 0.001), accounting for a total of 11.8% of the observed variance. The single strongest category was neural gene expression accounting for 3.1% of the variance.
Conclusions: The analysis of the relationship between the molecular genetic diagnoses of a hereditary etiology of hearing loss and cochlear implantation outcomes in a large German cohort of CI recipients revealed significant variabilities. Poor performance was observed with genetic mutations that affected the neural components of the cochlea, supporting the "spiral ganglion hypothesis."
Copyright © 2023 The Authors. Ear & Hearing is published on behalf of the American Auditory Society, by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Sprache für Gehörprüfung - Teil 1: Ein- und mehrsilbige Wörter (German). In G. I. f. S. R. n. c. D. G.-H. DIN, Audiometer und Kuppler (Ed.): Beuth Verlag GmbH.
-
- Abdurehim Y., Lehmann A., Zeitouni A. G. (2017). Predictive value of GJB2 mutation status for hearing outcomes of pediatric cochlear implantation. Otolaryngol Head Neck Surg, 157, 16–24. - PubMed
-
- Azaiez H., Booth K. T., Ephraim S. S., Crone B., Black-Ziegelbein E. A., Marini R. J., Shearer A. E., Sloan-Heggen C. M., Kolbe D., Casavant T., Schnieders M. J., Nishimura C., Braun T., Smith R. J. H. (2018). Genomic landscape and mutational signatures of deafness-associated genes. Am J Hum Genet, 103, 484–497. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

