Immunogenicity, safety and reactogenicity of heterologous (third dose) booster vaccination with a full or fractional dose of two different COVID-19 vaccines: A phase 4, single-blind, randomized controlled trial in adults
- PMID: 37438960
- PMCID: PMC10348030
- DOI: 10.1080/21645515.2023.2233400
Immunogenicity, safety and reactogenicity of heterologous (third dose) booster vaccination with a full or fractional dose of two different COVID-19 vaccines: A phase 4, single-blind, randomized controlled trial in adults
Abstract
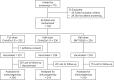
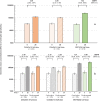
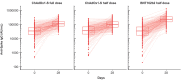
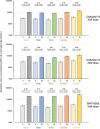
In this phase 4 study we assessed boosting with fractional doses of heterologous COVID-19 vaccines in Brazilian adults primed with two doses of CoronaVac (Sinovac/Butantan, São Paulo, Brazil) at least 4 months previously. Participants received either full-dose of ChAdOx1-S (Group 1, n = 232), a half dose of ChAdOx1-S (Group 2, n = 236), or a half dose of BNT162b2 (Group 3, n = 234). The primary objective was to show 80% seroresponse rates (SRR) 28 d after vaccination measured as IgG antibodies against a prototype SARS-CoV-2 spike-protein. Safety was assessed as solicited and unsolicited adverse events. At baseline all participants were seropositive, with high IgG titers overall. SRR at Day 28 were 34.3%, 27.1% and 71.2%, respectively, not meeting the primary objective of 80%, despite robust immune responses in all three groups with geometric mean-fold rise (GMFR) in IgG titers of 3.39, 2.99 and 7.42, respectively. IgG immune responses with similar GMFR were also observed against SARS-CoV-2 variants, Alpha, Beta, Delta, Gamma and D614G. In subsets (n = 35) of participants GMFR of neutralizing immune responses against live prototype SARS-CoV-2 virus and Omicron BA.2 were similar to the IgG responses as were pseudo-neutralizing responses against SARS-CoV-2 prototype and Omicron BA.4/5 variants. All vaccinations were well tolerated with no vaccine-related serious adverse events and mainly transient mild-to-moderate local and systemic reactogenicity. Heterologous boosting with full or half doses of ChAdOx1-S or a half dose of BNT162b2 was safe and immunogenic in CoronaVac-primed adults, but seroresponse rates were limited by high baseline immunity.
Keywords: BNT162b2; COVID-19; ChAdOx1-S; fractional dose; heterologous booster; neutralizing antibodies; vaccine.
Conflict of interest statement
A.J.P. is Chair of the UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in the JCVI COVID-19 committee and was a member of the WHO SAGE until 2022. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, or WHO. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
