Mid-term safety and efficacy of magnesium bioresorbable vascular scaffolds - magmaris in diabetic population. 2-Years outcome in acute coronary syndrome cohort
- PMID: 37439002
- PMCID: PMC10345934
- DOI: 10.1177/14791641231188705
Mid-term safety and efficacy of magnesium bioresorbable vascular scaffolds - magmaris in diabetic population. 2-Years outcome in acute coronary syndrome cohort
Abstract
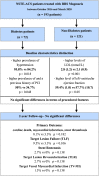
Background: Diabetes type 2 is one of the strongest risk factors affecting coronary artery disease (CAD) and is also a marker of poor short and long-term prognosis in subjects with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI) with subsequent drug-eluting stent (DES) implantation. Chronic local vascular inflammation along with endothelial dysfunction is postulated to be the pathophysiological background of unfavorable results. The second generation of metallic magnesium BRS -Magmaris (Biotronik, Berlin, Germany) had been introduced to clinical practice to overcome these limitations.
Methods: We evaluated 2-years clinical outcomes after Magmaris BRS implantation in NSTE-ACS diabetic (n-72) and non-diabetic (n-121) cohorts.
Results: No significant differences between diabetic and non-diabetes cohorts were noticed in terms of Primary Outcome (cardiac death, myocardial infarction, stent thrombosis) (8.1% vs 3.3% p = 0.182) and Principal secondary outcome - TLF- target lesion failure (9.5% vs 3.3% p = 0.106) at 2-years follow-up.
Conclusions: 2-years outcome suggests good safety and efficacy of the magnesium BRS (Magmaris) in NSTE- ACS and concomitant DM. Nevertheless, there is a strong need for large multicenter, randomized, prospective studies for a full assessment of this novel device in diabetic patients with ACS.
Keywords: Diabetes mellitus (DM); acute coronary syndrome (ACS); bioresorbable materials; coronary artery disease (CAD); magmaris; magnesium bioresorbable scaffold (BRS); mid-term outcome; percutaneous coronary intervention (PCI).
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Similar articles
-
Biodegradable Polymer DES (Ultimaster) vs. Magnesium Bioresorbable Scaffold (BRS Magmaris) in Diabetic Population with NSTE-ACS: A One-Year Clinical Outcome of Two Sirolimus-Eluting Stents.J Diabetes Res. 2021 Nov 23;2021:8636050. doi: 10.1155/2021/8636050. eCollection 2021. J Diabetes Res. 2021. PMID: 34859105 Free PMC article.
-
Early outcome of magnesium bioresorbable scaffold implantation in acute coronary syndrome-the initial report from the Magmaris-ACS registry.Catheter Cardiovasc Interv. 2019 Apr 1;93(5):E287-E292. doi: 10.1002/ccd.28036. Epub 2018 Dec 10. Catheter Cardiovasc Interv. 2019. PMID: 30537203
-
The 1-Year Safety and Efficacy Outcomes of Magmaris, Novel Magnesium Bioresorbable Vascular Scaffolds in Diabetes Mellitus Patients with Acute Coronary Syndrome.J Clin Med. 2021 Jul 18;10(14):3166. doi: 10.3390/jcm10143166. J Clin Med. 2021. PMID: 34300332 Free PMC article.
-
The bioresorbable magnesium scaffold (Magmaris)-State of the art: From basic concept to clinical application.Catheter Cardiovasc Interv. 2022 Nov;100(6):1051-1058. doi: 10.1002/ccd.30435. Epub 2022 Oct 13. Catheter Cardiovasc Interv. 2022. PMID: 36229949 Review.
-
Poly (l-lactic acid) bioresorbable scaffolds versus metallic drug-eluting stents for the treatment of coronary artery disease: A meta-analysis of 11 randomized trials.Catheter Cardiovasc Interv. 2020 Oct 1;96(4):813-824. doi: 10.1002/ccd.28594. Epub 2019 Nov 15. Catheter Cardiovasc Interv. 2020. PMID: 31730255
Cited by
-
Two-Year Outcomes for Patients with Non-ST-Elevation Acute Coronary Syndrome Treated with Magmaris and Absorb Bioresorbable Scaffolds in Large-Vessel Lesions.J Pers Med. 2024 May 17;14(5):540. doi: 10.3390/jpm14050540. J Pers Med. 2024. PMID: 38793122 Free PMC article.
-
Comparison of adverse cardiovascular event endpoints between patients with diabetes and patients without diabetes based on coronary artery plaques: a systematic review and meta-analysis.J Cardiothorac Surg. 2024 Dec 20;19(1):672. doi: 10.1186/s13019-024-03157-0. J Cardiothorac Surg. 2024. PMID: 39707525 Free PMC article.
References
-
- Arnold SV, Bhatt DL, Barsness GW, et al.American Heart Association Council on Lifestyle and Cardiometabolic Health and Council on Clinical Cardiology. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020; 141: e779–e806. - PubMed
-
- Naito R, Miyauchi K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int Heart J 2017; 58: 475–480. - PubMed
-
- Goyal SN, Bharti S, Krishnamurthy B, et al.Impact of metabolic syndrome on re-stenosis development: role of drug-eluting stents. Diab Vasc Dis Res 2012; 9: 177–188. - PubMed
-
- Giustino G, Colombo A, Camaj A, et al.Coronary In-Stent Restenosis: JACC State-of-the-Art Review. J Am Coll Cardiol 2022; 80: 348–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous