Population-level impact of expanding PrEP coverage by offering long-acting injectable PrEP to MSM in three high-resource settings: a model comparison analysis
- PMID: 37439080
- PMCID: PMC10339001
- DOI: 10.1002/jia2.26109
Population-level impact of expanding PrEP coverage by offering long-acting injectable PrEP to MSM in three high-resource settings: a model comparison analysis
Abstract
Introduction: Long-acting injectable cabotegravir (CAB-LA) demonstrated superiority to daily tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for HIV pre-exposure prophylaxis (PrEP) in the HPTN 083/084 trials. We compared the potential impact of expanding PrEP coverage by offering CAB-LA to men who have sex with men (MSM) in Atlanta (US), Montreal (Canada) and the Netherlands, settings with different HIV epidemics.
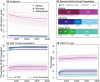
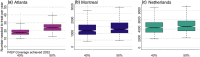
Methods: Three risk-stratified HIV transmission models were independently parameterized and calibrated to local data. In Atlanta, Montreal and the Netherlands, the models, respectively, estimated mean TDF/FTC coverage starting at 29%, 7% and 4% in 2022, and projected HIV incidence per 100 person-years (PY), respectively, decreasing from 2.06 to 1.62, 0.08 to 0.03 and 0.07 to 0.001 by 2042. Expansion of PrEP coverage was simulated by recruiting new CAB-LA users and by switching different proportions of TDF/FTC users to CAB-LA. Population effectiveness and efficiency of PrEP expansions were evaluated over 20 years in comparison to baseline scenarios with TDF/FTC only.
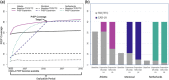
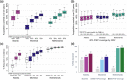
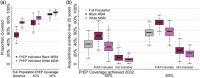
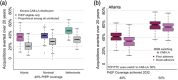
Results: Increasing PrEP coverage by 11 percentage points (pp) from 29% to 40% by 2032 was expected to avert a median 36% of new HIV acquisitions in Atlanta. Substantially larger increases (by 33 or 26 pp) in PrEP coverage (to 40% or 30%) were needed to achieve comparable reductions in Montreal and the Netherlands, respectively. A median 17 additional PYs on PrEP were needed to prevent one acquisition in Atlanta with 40% PrEP coverage, compared to 1000+ in Montreal and 4000+ in the Netherlands. Reaching 50% PrEP coverage by 2032 by recruiting CAB-LA users among PrEP-eligible MSM could avert >45% of new HIV acquisitions in all settings. Achieving targeted coverage 5 years earlier increased the impact by 5-10 pp. In the Atlanta model, PrEP expansions achieving 40% and 50% coverage reduced differences in PrEP access between PrEP-indicated White and Black MSM from 23 to 9 pp and 4 pp, respectively.
Conclusions: Achieving high PrEP coverage by offering CAB-LA can impact the HIV epidemic substantially if rolled out without delays. These PrEP expansions may be efficient in settings with high HIV incidence (like Atlanta) but not in settings with low HIV incidence (like Montreal and the Netherlands).
Keywords: Europe; HIV prevention; North America; PrEP; men who have sex with men; modelling.
© 2023 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Estimating the impact of HIV PrEP regimens containing long-acting injectable cabotegravir or daily oral tenofovir disoproxil fumarate/emtricitabine among men who have sex with men in the United States: a mathematical modelling study for HPTN 083.Lancet Reg Health Am. 2023 Jan 17;18:100416. doi: 10.1016/j.lana.2022.100416. eCollection 2023 Feb. Lancet Reg Health Am. 2023. PMID: 36844011 Free PMC article.
-
Estimated impact of long-acting injectable PrEP in South Africa: a model comparison analysis.J Int AIDS Soc. 2025 Jul;28 Suppl 2(Suppl 2):e26453. doi: 10.1002/jia2.26453. J Int AIDS Soc. 2025. PMID: 40600502 Free PMC article.
-
Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States : A Cost-Effectiveness Analysis.Ann Intern Med. 2022 Apr;175(4):479-489. doi: 10.7326/M21-1548. Epub 2022 Feb 1. Ann Intern Med. 2022. PMID: 35099992 Free PMC article.
-
Global HIV Incidence Analysis and Implications for Affordability Using Long-Acting Cabotegravir Versus Continuous and Event-Driven Oral Preexposure Prophylaxis.Clin Infect Dis. 2024 Feb 17;78(2):386-394. doi: 10.1093/cid/ciad537. Clin Infect Dis. 2024. PMID: 37665213 Free PMC article.
-
Preexposure Prophylaxis for the Prevention of HIV: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2023 Aug 22;330(8):746-763. doi: 10.1001/jama.2023.9865. JAMA. 2023. PMID: 37606667
Cited by
-
Epidemiological impact and cost-effectiveness analysis of PrEP provision expansion among MSM in the Netherlands.J Int AIDS Soc. 2025 Jun;28(6):e26516. doi: 10.1002/jia2.26516. J Int AIDS Soc. 2025. PMID: 40462500 Free PMC article.
-
Long-acting preexposure prophylaxis: early data on roll-out in the United States.Curr Opin HIV AIDS. 2025 Jan 1;20(1):25-31. doi: 10.1097/COH.0000000000000894. Epub 2024 Nov 5. Curr Opin HIV AIDS. 2025. PMID: 39633537 Review.
-
Brief Report: Interest in Long-acting Injectable PrEP Among Transgender Women in Eastern and Southern United States.J Acquir Immune Defic Syndr. 2024 Sep 1;97(1):19-25. doi: 10.1097/QAI.0000000000003465. J Acquir Immune Defic Syndr. 2024. PMID: 40788229 Free PMC article.
-
Advancing use of long-acting and extended delivery HIV prevention and treatment regimens.J Int AIDS Soc. 2023 Jul;26 Suppl 2(Suppl 2):e26126. doi: 10.1002/jia2.26126. J Int AIDS Soc. 2023. PMID: 37439079 Free PMC article. No abstract available.
-
The Disparities of PrEP Adherence Among Men Who Have Sex With Men Between the Global South and the Global North: An Updated Determinantal Global Meta-Analysis.J Acquir Immune Defic Syndr. 2025 May 1;99(1):1-8. doi: 10.1097/QAI.0000000000003635. J Acquir Immune Defic Syndr. 2025. PMID: 39878582 Free PMC article.
References
-
- Kerrigan D, Mantsios A, Grant R, Markowitz M, Defechereux P, La Mar M, et al. Expanding the menu of HIV prevention options: a qualitative study of experiences with long‐acting injectable cabotegravir as PrEP in the context of a phase II trial in the United States. AIDS Behav. 2018;. 22(11):3540–9. - PMC - PubMed
-
- Murray MI, Markowitz M, Frank I, Grant RM, Mayer KH, Hudson KJ, et al. Satisfaction and acceptability of cabotegravir long‐acting injectable suspension for prevention of HIV: patient perspectives from the ECLAIR trial. HIV Clin Trials. 2018;. 19(4):129–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

