Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: a multicenter, retrospective, real-world experience with 200 cases outside of controlled clinical trials
- PMID: 37439329
- PMCID: PMC10772491
- DOI: 10.3324/haematol.2023.283251
Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: a multicenter, retrospective, real-world experience with 200 cases outside of controlled clinical trials
Abstract
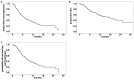
In the ELOQUENT-3 trial, the combination of elotuzumab, pomalidomide and dexamethasone (EloPd) proved to have a superior clinical benefit over pomalidomide and dexamethasone with a manageable toxicity profile, leading to its approval for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor. We report here a real-world experience of 200 cases of RRMM treated with EloPd in 35 Italian centers outside of clinical trials. In our dataset, the median number of prior lines of therapy was two, with 51% of cases undergoing autologous stem cell transplant and 73% having been exposed to daratumumab. After a median follow-up of 9 months, 126 patients had stopped EloPd, most of them (88.9%) because of disease progression. The overall response rate was 55.4%, a finding in line with the pivotal trial results. Regarding adverse events, the toxicity profile in our cohort was similar to that in the ELOQUENT-3 trial, with no significant differences between younger (<70 years) and older patients. The median progression-free survival was 7 months, which was shorter than that observed in ELOQUENT-3, probably because of the different clinical characteristics of the two cohorts. Interestingly, International Staging System stage III disease was associated with worse progression-free survival (hazard ratio=2.55). Finally, the median overall survival of our series was shorter than that observed in the ELOQUENT-3 trial (17.5 vs. 29.8 months). In conclusion, our real-world study confirms that EloPd is a safe and possible therapeutic choice for patients with RRMM who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
Figures
References
-
- Nooka AK, Kaufman JL, Hofmeister CC, et al. . Daratumumab in multiple myeloma. Cancer. 2019;125(14):2364-2382. - PubMed
-
- Bruzzese A, Martino EA, Vigna E, et al. . Elotuzumab in multiple myeloma. Expert Opin Biol Ther. 2023;23(1):7-10. - PubMed
-
- Durer C, Durer S, Lee S, et al. . Treatment of relapsed multiple myeloma: evidence-based recommendations. Blood Rev. 2020;39:100616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

