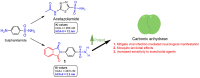
Carbonic anhydrase inhibitory activity of phthalimide-capped benzene sulphonamide derivatives
- PMID: 37439360
- PMCID: PMC10348020
- DOI: 10.1080/14756366.2023.2235089
Carbonic anhydrase inhibitory activity of phthalimide-capped benzene sulphonamide derivatives
Abstract
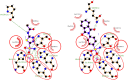
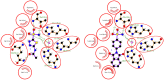
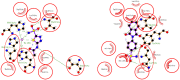
A series of phthalimide-capped benzene sulphonamides (1-22) reported by our group for dengue protease inhibitory activity have been evaluated for their carbonic anhydrase (hCA, EC 4.2.1.1) inhibitory activity against hCA I, hCA II. Compounds 1, 3, 10, and 15 showed hCA I inhibition, whereas 1, 4, and 10 showed hCA II inhibition at nanomolar concentrations. Among these compounds, 1 displayed potent inhibitory activity against the hCA I (Ki = 28.5 nM) and hCA II (Ki = 2.2 nM), being 10 and 6 times more potent than acetazolamide, a standard inhibitor (Ki = 250 nM and 12 nM), respectively. Furthermore, this compound displayed 14-fold selectivity towards the hCA II isoform compared to hCA I. Molecular docking and MD simulations were performed to understand the atomic level interactions responsible for the selectivity of compound 1 towards hCA II.
Keywords: Carbonic anhydrase; molecular docking; molecular dynamics; phthalyl sulphamoyl derivatives.
Conflict of interest statement
All authors (except CTS) declare no conflict of interest. CT Supuran is Editor-in-Chief of the
Figures
Similar articles
-
New anthranilic acid-incorporating N-benzenesulfonamidophthalimides as potent inhibitors of carbonic anhydrases I, II, IX, and XII: Synthesis, in vitro testing, and in silico assessment.Eur J Med Chem. 2019 Nov 1;181:111573. doi: 10.1016/j.ejmech.2019.111573. Epub 2019 Aug 1. Eur J Med Chem. 2019. PMID: 31394463
-
Discovery of novel benzenesulfonamides incorporating 1,2,3-triazole scaffold as carbonic anhydrase I, II, IX, and XII inhibitors.Int J Biol Macromol. 2023 Jun 1;239:124232. doi: 10.1016/j.ijbiomac.2023.124232. Epub 2023 Mar 30. Int J Biol Macromol. 2023. PMID: 37001773
-
Synthesis, carbonic anhydrase inhibition studies and modelling investigations of phthalimide-hydantoin hybrids.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2335927. doi: 10.1080/14756366.2024.2335927. Epub 2024 Apr 12. J Enzyme Inhib Med Chem. 2024. PMID: 38606915 Free PMC article.
-
Interactions of novel 1,3-diaryltriazene-sulfamethazines with carbonic anhydrases: Kinetic studies and in silico simulations.Arch Biochem Biophys. 2024 Nov;761:110181. doi: 10.1016/j.abb.2024.110181. Epub 2024 Oct 11. Arch Biochem Biophys. 2024. PMID: 39396797
-
Exploration of 2-phenylquinoline-4-carboxamide linked benzene sulfonamide derivatives as isoform selective inhibitors of transmembrane human carbonic anhydrases.Eur J Med Chem. 2022 Apr 15;234:114247. doi: 10.1016/j.ejmech.2022.114247. Epub 2022 Mar 9. Eur J Med Chem. 2022. PMID: 35305463
Cited by
-
Prospects for Prostate Cancer Chemotherapy: Cytotoxic Evaluation and Mechanistic Insights of Quinolinequinones with ADME/PK Profile.Biomedicines. 2024 Jun 3;12(6):1241. doi: 10.3390/biomedicines12061241. Biomedicines. 2024. PMID: 38927448 Free PMC article.
-
Synthesis, In Vivo Anticonvulsant Activity Evaluation and In Silico Studies of Some Quinazolin-4(3H)-One Derivatives.Molecules. 2024 Apr 24;29(9):1951. doi: 10.3390/molecules29091951. Molecules. 2024. PMID: 38731442 Free PMC article.
-
In silico evaluation of a new compound incorporating 4(3H)-quinazolinone and sulfonamide as a potential inhibitor of a human carbonic anhydrase.BMC Chem. 2024 Mar 4;18(1):45. doi: 10.1186/s13065-024-01150-1. BMC Chem. 2024. PMID: 38433188 Free PMC article.
-
Computational methods in glaucoma research: Current status and future outlook.Mol Aspects Med. 2023 Dec;94:101222. doi: 10.1016/j.mam.2023.101222. Epub 2023 Nov 3. Mol Aspects Med. 2023. PMID: 37925783 Free PMC article. Review.
References
-
- Agamennone M, Fantacuzzi M, Carradori S, Petzer A, Petzer JP, Angeli A, Supuran CT, Luisi G.. Coumarin-based dual inhibitors of human carbonic anhydrases and monoamine oxidases featuring amino acyl and (pseudo)-dipeptidyl appendages: in vitro and computational studies. Molecules. 2022;27(22):7884. - PMC - PubMed
-
- Kumar S, Rulhania S, Jaswal S, Monga V.. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur J Med Chem. 2021;209(:112923. - PubMed
-
- Arechederra RL, Waheed A, Sly WS, Supuran CT, Minteer SD.. Effect of sulfonamides as carbonic anhydrase va and vb inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem. 2013;21(6):1544–1548. - PubMed
-
- Gawad NMA, Amin NH, Elsaadi MT, Mohamed FMM, Angeli A, De Luca V, Capasso C, Supuran CT.. Synthesis of 4-(thiazol-2-ylamino)-benzenesulfonamides with carbonic anhydrase i, ii and ix inhibitory activity and cytotoxic effects against breast cancer cell lines. Bioorg Med Chem. 2016;24(13):3043–3051. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
