Absence of E-Cadherin and β-Catenin in the Basal Plasma Membrane of Collecting Duct Cells During NDI Development and Recovery
- PMID: 37439659
- PMCID: PMC10363910
- DOI: 10.1369/00221554231185809
Absence of E-Cadherin and β-Catenin in the Basal Plasma Membrane of Collecting Duct Cells During NDI Development and Recovery
Abstract
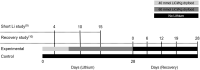
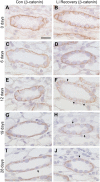
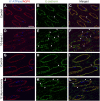
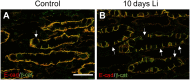
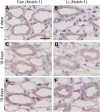
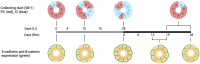
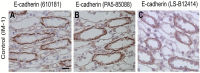
Lithium (Li) induces severe polyuria and polydipsia in up to 40% of patients undergoing Li treatment. In rats, Li treatment induces a reversible cellular remodeling of the collecting duct (CD), decreasing the fraction of principal-to-intercalated cells. To investigate the potential role of adherens junction proteins, we performed immunohistochemistry on kidney cross-sections from rats treated with Li as well as rats undergoing recovery on a normal diet following 4 weeks of Li-treatment. We performed immunoelectron microscopy on cryosections to determine the ultrastructural localizations. Immunohistochemistry showed that E-cadherin and β-catenin were present in both the lateral and basal plasma membrane domains of CD cells. Immunoelectron microscopy confirmed that β-catenin was localized both to the lateral and the basal plasma membrane. The basal localization of both proteins was absent from a fraction of mainly principal cells after 10 and 15 days of Li-treatment. After 4 weeks of Li-treatment few to no cells were absent of E-cadherin and β-catenin at the basal plasma membrane. After 12 and 19 days of recovery some cells exhibited an absence of basal localization of both proteins. Thus, the observed localizational changes of E-cadherin and β-catenin appear before the cellular remodeling during both development and recovery from Li-NDI.
Keywords: adherens junction; cellular remodeling; collecting duct; diabetes insipidus; lithium.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures








Similar articles
-
Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI.Am J Physiol Cell Physiol. 2004 Apr;286(4):C952-64. doi: 10.1152/ajpcell.00266.2003. Epub 2003 Nov 12. Am J Physiol Cell Physiol. 2004. PMID: 14613889
-
Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct.Am J Physiol Renal Physiol. 2006 Jul;291(1):F39-48. doi: 10.1152/ajprenal.00383.2005. Epub 2006 Jan 24. Am J Physiol Renal Physiol. 2006. PMID: 16434572
-
Pathogenesis of nephrogenic diabetes insipidus due to chronic administration of lithium in rats.J Clin Invest. 1985 Jun;75(6):1869-79. doi: 10.1172/JCI111901. J Clin Invest. 1985. PMID: 2989335 Free PMC article.
-
Distribution of adherens junction mediated by VE-cadherin complex in rat spleen sinus endothelial cells.Cell Tissue Res. 2006 Mar;323(3):417-24. doi: 10.1007/s00441-005-0064-5. Epub 2005 Oct 22. Cell Tissue Res. 2006. PMID: 16244888
-
Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings.J Nephrol. 2010 Nov-Dec;23 Suppl 16:S43-8. J Nephrol. 2010. PMID: 21170888 Review.
Cited by
-
Lithium Ions as Modulators of Complex Biological Processes: The Conundrum of Multiple Targets, Responsiveness and Non-Responsiveness, and the Potential to Prevent or Correct Dysregulation of Systems during Aging and in Disease.Biomolecules. 2024 Jul 25;14(8):905. doi: 10.3390/biom14080905. Biomolecules. 2024. PMID: 39199293 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

