Testing for Human Papillomaviruses in Urine, Blood, and Oral Specimens: an Update for the Laboratory
- PMID: 37439692
- PMCID: PMC10446865
- DOI: 10.1128/jcm.01403-22
Testing for Human Papillomaviruses in Urine, Blood, and Oral Specimens: an Update for the Laboratory
Abstract
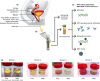
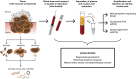
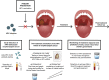
Twelve high-risk alpha human papillomavirus (HPV) genotypes cause approximately 690,000 cancer cases annually, with cervical and oropharyngeal cancer being the two most prominent types. HPV testing is performed in laboratory settings for various applications of a clinical, epidemiological, and research nature using a range of clinical specimens collected by clinicians or by individuals (self-collected specimens). Here, we reflect on the importance and justification of using the right test for the right application and provide practical updates for laboratories either participating in or anticipating involvement in HPV testing in three specimen types, namely, urine, blood, and oral specimens, which are considered "alternative" specimens by many. In addition to clinician-collected cervical samples and self-collected cervicovaginal samples, first-void urine is emerging as a credible specimen for HPV-based cervical cancer screening, triage of HPV screen-positive women, monitoring HPV vaccine impact, and HPV testing in groups for which a less invasive sample is preferred. Detection of cell-free DNA (including HPV DNA) in blood has great promise for the early detection of HPV-attributable oropharyngeal cancer (HPV-AOC) and potentially other HPV-driven cancers and as an adjunct prognostic marker in long-term tumor surveillance, including treatment response. The moderate sensitivity of HPV testing in oral rinses or swabs at HPV-AOC diagnosis prevents its use in HPV-AOC secondary prevention but represents a promising prognostic tool in HPV-AOC tertiary prevention, where the HPV persistence in oral rinses throughout treatment may predict early HPV-AOC recurrences and/or the development of secondary HPV-AOC. The increasing sophistication of specific collection devices designed for alternative samples and the enhanced precision of novel molecular technologies are likely to support the evolution of this field and catalyze potential translation into routine practice.
Keywords: HPV; blood; cervical cancer; oral specimens; oropharyngeal cancer; urine.
Conflict of interest statement
The authors declare a conflict of interest. M.P. institution received research funding, free-of-charge reagents, and consumables to support research in the last 3 years from Qiagen, Seegene, Abbott, and Roche, all paid to his employer. K.C. institution received research funding, reagents, and consumables to support research in the last 3 years from Cepheid, Euroimmun, GeneFirst, Self-screen, Hiantis, Seegene, Roche, Abbott, Hologic, and Vaccitech, all paid to her employer. K.C. also attended an advisory board meeting of Hologic where UK-based travel expenses were supported and is currently on the advisory board of Vaccitech (with any associated reimbursement paid to employer). L.A. institution received funding to support research in the last 3 years from Merck Sharp & Dohme, Roche, GSK, Vitro, Hologic, and Seegene, all paid to her employer. A.V. institution received funding to support research and setting up professional meetings in the last 3 years from Merck, Roche, GSK, Hologic, Abbott, Novosanis and Becton, Dickinson and Company, all paid to his employer. A.V. is the co-founder of Novosanis, a spin-off company of the University of Antwerp, Belgium, and a subsidiary of OraSure Technologies, Inc. since 2019, and he was a board member and minority shareholder until January 2019.
Figures