Treatment With Niraparib Maintenance Therapy in Patients With Newly Diagnosed Advanced Ovarian Cancer: A Phase 3 Randomized Clinical Trial
- PMID: 37440217
- PMCID: PMC10346505
- DOI: 10.1001/jamaoncol.2023.2283
Treatment With Niraparib Maintenance Therapy in Patients With Newly Diagnosed Advanced Ovarian Cancer: A Phase 3 Randomized Clinical Trial
Abstract
Importance: The efficacy of niraparib maintenance therapy with an individualized starting dose (ISD) warrants further investigation in a broad population with newly diagnosed advanced ovarian cancer (aOC), including patients without postoperative residual disease.
Objective: To evaluate the efficacy and safety of niraparib with an ISD in a broad population with newly diagnosed aOC (R0 resection permitted).
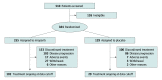
Design, setting, and participants: This multicenter, randomized, double-blind, placebo-controlled, phase 3 study was conducted in China and enrolled 384 patients with newly diagnosed aOC who received primary or interval debulking surgery and responded to treatment with first-line platinum-based chemotherapy. By data cutoff (September 30, 2021), median follow-up for progression-free survival (PFS) was 27.5 (IQR, 24.7-30.4) months.
Interventions: Patients were randomized 2:1 to receive niraparib or placebo with ISD (200 mg/d for those with a body weight of <77 kg and/or platelet count of <150 ×103/μL [to convert to ×109/μL, multiply by 1] at baseline; 300 mg/d otherwise) stratified by germline BRCA variant status, tumor homologous recombination deficiency status, neoadjuvant chemotherapy, and response to first-line platinum-based chemotherapy.
Main outcomes and measurements: The primary end point was blinded, independent central review-assessed PFS in the intention-to-treat population.
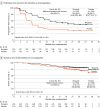
Results: A total of 384 patients were randomized (255 niraparib [66.4%]; median [range] age, 53 [32-77] years; 129 placebo [33.6%]; median [range] age, 54 [33-77] years), and 375 (247 niraparib [65.9%], 128 placebo [34.1%]) received treatment at a dose of 200 mg per day. Median PFS with niraparib vs placebo was 24.8 vs 8.3 months (hazard ratio [HR], 0.45; 95% CI, 0.34-0.60; P < .001) in the intention-to-treat population; not reached vs 10.8 months (HR, 0.40; 95% CI, 0.23-0.68) and 19.3 vs 8.3 months (HR, 0.48; 95% CI, 0.34-0.67) in patients with and without germline BRCA variants, respectively; not reached vs 11.0 months (HR, 0.48; 95% CI, 0.34-0.68) and 16.6 vs 5.5 months (HR, 0.41; 95% CI, 0.22-0.75) in homologous recombination deficient and proficient patients, respectively; and 24.8 vs 8.3 months (HR, 0.44; 95% CI, 0.32-0.61) and 16.5 vs 8.3 months (HR, 0.27; 95% CI, 0.10-0.72) in those with optimal and suboptimal debulking, respectively. Similar proportions of niraparib-treated and placebo-treated patients (6.7% vs 5.4%) discontinued treatment due to treatment-emergent adverse events.
Conclusion and relevance: This randomized clinical trial found that niraparib maintenance therapy prolonged PFS in patients with newly diagnosed aOC regardless of postoperative residual disease or biomarker status. The ISD was effective and safe in the first-line maintenance setting.
Trial registration: ClinicalTrials.gov Identifier: NCT03709316.
Conflict of interest statement
Figures

References
-
- Gadducci A, Cosio S. Randomized clinical trials and real world prospective observational studies on bevacizumab, PARP inhibitors, and immune checkpoint inhibitors in the first-line treatment of advanced ovarian carcinoma: a critical review. Anticancer Res. 2021;41(10):4673-4685. doi:10.21873/anticanres.15281 - DOI - PubMed

