Efficacy of Metronomic Oral Vinorelbine, Cyclophosphamide, and Capecitabine vs Weekly Intravenous Paclitaxel in Patients With Estrogen Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: Final Results From the Phase 2 METEORA-II Randomized Clinical Trial
- PMID: 37440239
- PMCID: PMC10346502
- DOI: 10.1001/jamaoncol.2023.2150
Efficacy of Metronomic Oral Vinorelbine, Cyclophosphamide, and Capecitabine vs Weekly Intravenous Paclitaxel in Patients With Estrogen Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: Final Results From the Phase 2 METEORA-II Randomized Clinical Trial
Abstract
Importance: In spite of the effectiveness of endocrine therapy plus cyclin-dependent kinase (CDK) 4/6 inhibitors as the first-line treatment for estrogen receptor (ER)-positive, erb-b2 receptor tyrosine kinase 2 (ERBB2 [formerly HER2/neu])-negative (ER+/ERBB2-) metastatic breast cancer (MBC), patients eventually develop resistance, and eventually most will receive chemotherapy. The METEORA-II trial compared a metronomic all-oral treatment with intravenous (IV) chemotherapy.
Objective: To compare the efficacy of the oral vinorelbine plus cyclophosphamide plus capecitabine (VEX) regimen vs weekly IV paclitaxel among patients with ER+/ERBB2- MBC who are candidates for chemotherapy.
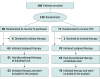
Design, setting, and participants: This phase 2 randomized clinical trial including 140 women 18 years and older (randomized 1:1) with ER+/ERBB2- MBC was carried out from September 13, 2017, to January 14, 2021 at 15 centers in Italy. Eligible patients could have received 1 prior line of chemotherapy for MBC and/or 2 lines of endocrine therapy (including CDK4/6 inhibitors).
Interventions: In 4-week cycles, patients received either metronomic oral VEX or weekly IV paclitaxel.
Main outcomes and measures: The primary end point was investigator-assessed time to treatment failure (TTF) defined as the interval between the date of randomization to the end of treatment (because of disease progression or lack of tolerability or because further trial treatment was declined). Secondary end points included progression-free survival (PFS), overall survival (OS), and disease control rate (complete or partial response or stable disease lasting for at least 24 weeks).
Results: In total, 133 patients received either VEX (n = 70) or paclitaxel (n = 63) in 4-weekly cycles. The median age was 61 (range, 30-80) years. The VEX treatment significantly prolonged TTF vs paclitaxel (hazard ratio [HR], 0.61; 95% CI, 0.42-0.88; P = .008), median TTF was 8.3 (95% CI, 5.6-11.1) months for VEX vs 5.7 (95% CI, 4.1-6.1) months for paclitaxel, and the 12-month TTF was 34.3% for VEX vs 8.6% for paclitaxel. The median PFS was 11.1 (95% CI, 8.3-13.8) months vs 6.9 (95% CI, 5.4-10.1) months favoring VEX (HR, 0.67; 95% CI, 0.46-0.96, P = .03). The 12-month PFS was 43.5% for VEX vs 21.9% for paclitaxel. No difference in OS was found. The TF event for 55.6% of patients was progression of disease; for 23% it was AEs. More patients assigned to VEX had at least 1 grade 3 or 4 targeted adverse event (VEX, 42.9%; 95% CI, 31.1%-55.3% vs paclitaxel, 28.6%; 95% CI, 17.9%-41.3%), but essentially no alopecia.
Conclusion and relevance: This randomized clinical trial found significantly prolonged TTF and PFS for oral VEX but no improvement in OS compared with intravenous paclitaxel, despite increased but still manageable toxic effects. The VEX regimen may provide more prolonged disease control than weekly paclitaxel for ER+/ERBB2- MBC.
Trial registration: ClinicalTrials.gov Identifier: NCT02954055.
Conflict of interest statement
Figures

References
-
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 (suppl 7). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

