COVID-19 Severity and Waning Immunity After up to 4 mRNA Vaccine Doses in 73 608 Patients With Cancer and 621 475 Matched Controls in Singapore: A Nationwide Cohort Study
- PMID: 37440245
- PMCID: PMC10346511
- DOI: 10.1001/jamaoncol.2023.2271
COVID-19 Severity and Waning Immunity After up to 4 mRNA Vaccine Doses in 73 608 Patients With Cancer and 621 475 Matched Controls in Singapore: A Nationwide Cohort Study
Abstract
Importance: Despite patients with cancer being at risk of poor outcomes from COVID-19, there are few published studies for vaccine efficacy in this group, with suboptimal immunogenicity and waning vaccine efficacy described in small studies being a concern.
Objective: To assess the incidence rate of severe COVID-19 disease outcomes associated with the number of vaccine doses received and the waning of protection over time.
Design, setting, and participants: A prospective multicenter observational cohort study was carried out over 2 time periods (September 15, 2021, to December 20, 2021 [delta wave], and January 20, 2022, to November 11, 2022 [omicron wave]) predominated by SARS-CoV-2 delta and omicron variants, respectively. Overall, 73 608 patients with cancer (23 217 active treatment, 50 391 cancer survivors) and 621 475 controls matched by age, sex, race and ethnicity, and socioeconomic status were included.
Exposure: Vaccine doses received, from zero to 4 doses, and time elapsed since last vaccine dose.
Outcomes: Competing-risk regression analyses were employed to account for competing risks of death in patients with cancer. Main outcomes were incidence rate ratios (IRRs) of COVID-19 infection, hospitalization, and severe disease (defined as requirement for supplemental oxygen, intensive care, or death). The IRRs stratified by time from last vaccine dose served as indicators of waning of vaccine effectiveness over time.
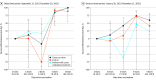
Results: The mean (SD) age of actively treated patients with cancer, cancer survivors, and controls were 62.7 (14.7), 62.9 (12.6), and 61.8 (14.7) years, respectively. Of 73 608 patients with cancer, 27 170 (36.9%) were men; 60 100 (81.6%) were Chinese, 7432 (10.1%) Malay, 4597 (6.2%) Indian, and 1479 (2.0%) were of other races and ethnicities. The IRRs for the 3-dose and 4-dose vs the 2-dose group (reference) for COVID-19 hospitalization and severe disease were significantly lower during both the delta and omicron waves in cancer and control populations. The IRRs for severe disease in the 3-dose group for active treatment, cancer survivors, and controls were 0.14, 0.13, and 0.07 during the delta wave and 0.29, 0.19, and 0.21 during omicron wave, respectively. The IRRs for severe disease in the 4-dose group during the omicron wave were even lower at 0.13, 0.10 and 0.10, respectively. No waning of vaccine effectiveness against hospitalization and severe disease was seen beyond 5 months after a third dose, nor up to 5 months (the end of this study's follow-up) after a fourth dose.
Conclusion: This cohort study provides evidence of the clinical effectiveness of mRNA-based vaccines against COVID-19 in patients with cancer. Longevity of immunity in preventing severe COVID-19 outcomes in actively treated patients with cancer, cancer survivors, and matched controls was observed at least 5 months after the third or fourth dose.
Conflict of interest statement
Figures
References
-
- Johns Hopkins University Coronavirus Resource Center . COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020. Accessed December 30, 2022. https://coronavirus.jhu.edu/map.html
-
- World Health Organization . WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. Accessed December 30, 2022. https://www.who.int/director-general/speeches/detail/who-director-genera...-the-media-briefing-on-cov
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

