Increasing challenges to trial recruitment and conduct over time
- PMID: 37440282
- PMCID: PMC10592378
- DOI: 10.1111/epi.17716
Increasing challenges to trial recruitment and conduct over time
Abstract
Objective: This study was undertaken to evaluate how the challenges in the recruitment and retention of participants in clinical trials for focal onset epilepsy have changed over time.
Methods: In this systematic analysis of randomized clinical trials of adjunct antiseizure medications for medication-resistant focal onset epilepsy, we evaluated how the numbers of participants, sites, and countries have changed since the first such trial in 1990. We also evaluated the proportion of participants who completed each trial phase and their reasons for early trial exit. We analyzed these trends using mixed effects generalized linear models accounting for the influence of the number of trial sites and trial-specific variability.
Results: The number of participants per site has steadily decreased over decades, with recent trials recruiting fewer than five participants per site (reduction by .16 participants/site/year, p < .0001). Fewer participants also progressed from recruitment to randomization over time (odds ratio = .94/year, p = .014). Concurrently, there has been an increase in the placebo response over time (increase in median percent reduction of .4%/year, p = .02; odds ratio of increase in 50% responder rate of 1.03/year, p = .02), which was not directly associated with the number of sites per trial (p > .20).
Significance: This historical analysis highlights the increasing challenges with participant recruitment and retention, as well as increasing placebo response. It serves as a call to action to change clinical trial design to address these challenges.
Keywords: antiepileptic drugs; clinical trials; epilepsy; time to event.
© 2023 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Figures

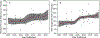

References
-
- Kwan P, et al., Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia, 2010. 51(6): p. 1069–77. - PubMed
-
- Kwan P and Brodie MJ, Early identification of refractory epilepsy. N Engl J Med, 2000. 342(5): p. 314–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

